3-乙酰基吡啶 | 350-03-8
物质功能分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:11-13 °C (lit.)
-
沸点:220 °C (lit.)
-
密度:1.102 g/mL at 25 °C (lit.)
-
闪点:302 °F
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
-
LogP:0.34 at 13℃
-
物理描述:Liquid
-
亨利常数:2.13e-08 atm-m3/mole
-
折光率:1.530-1.540
-
保留指数:1128;1075.7
-
稳定性/保质期:
- 该物质有毒,具有刺激性,可能通过口服或皮肤接触对人体造成伤害。
计算性质
-
辛醇/水分配系数(LogP):0.4
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:30
-
氢给体数:0
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险等级:6.1
-
危险品标志:T
-
安全说明:S26,S28A,S37/39,S45
-
危险类别码:R25,R36/38
-
WGK Germany:3
-
海关编码:29333999
-
危险品运输编号:UN 2810 6.1/PG 3
-
危险类别:6.1
-
RTECS号:OB5425000
-
包装等级:II
-
危险标志:GHS06
-
危险性描述:H301
-
危险性防范说明:P301 + P310
-
储存条件:密封避光保存。
SDS
模块 1. 化学品
1.1 产品标识符
: 3-乙酰基吡啶
产品名称
1.2 鉴别的其他方法
Methyl 3-pyridyl ketone
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
急性毒性, 经口 (类别 3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 危险
危险申明
H301 吞咽会中毒
警告申明
预防措施
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
事故响应
P301 + P310 如果吞下去了: 立即呼救解毒中心或医生。
P321 具体处置(见本标签上提供的急救指导)。
P330 漱口。
安全储存
P405 存放处须加锁。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: Methyl 3-pyridyl ketone
别名
: C7H7NO
分子式
: 121.14 g/mol
分子量
组分 浓度或浓度范围
Methyl 3-pyridyl ketone
<=100%
化学文摘登记号(CAS 350-03-8
No.) 206-496-7
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 立即将患者送往医院。 请教医生。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
戴呼吸罩。 避免吸入蒸气、烟雾或气体。 保证充分的通风。 人员疏散到安全区域。
6.2 环境保护措施
如能确保安全,可采取措施防止进一步的泄漏或溢出。 不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
用惰性吸附材料吸收并当作危险废物处理。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免吸入蒸气和烟雾。
一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
避免与皮肤、眼睛和衣服接触。 休息前和操作本品后立即洗手。
个体防护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
物料: 丁基橡胶
最小的层厚度 0.3 mm
溶剂渗透时间: 480 min
测试过的物质ButojECt® (KCL 897 / Z677647, 规格 M)
飞溅保护
物料: 丁基橡胶
最小的层厚度 0.3 mm
溶剂渗透时间: 480 min
测试过的物质ButojECt® (KCL 897 / Z677647, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 透明, 液体
颜色: 深黄
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 11 - 13 °C - lit.
f) 沸点、初沸点和沸程
220 °C - lit.
g) 闪点
104 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
1.102 g/cm3 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂, 强酸, 强还原剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 经口 - 大鼠 - 50.9 mg/kg
备注: 行为的:嗜睡(全面活力抑制)。 行为的:抽搐或对癫痫阈值的影响。 其他改变
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
从实验动物的结果看,过度接触能导致生殖紊乱
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 误吞会中毒。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: OB5425000
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
联系专业的拥有废弃物处理执照的机构来处理此物质。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: 2810 国际海运危规: 2810 国际空运危规: 2810
14.2 联合国运输名称
欧洲陆运危规: TOXIC LIQUID, ORGANIC, N.O.S. (Methyl 3-pyridyl ketone)
国际海运危规: TOXIC LIQUID, ORGANIC, N.O.S. (Methyl 3-pyridyl ketone)
国际空运危规: Toxic liquid, organic, n.o.s. (Methyl 3-pyridyl ketone)
14.3 运输危险类别
欧洲陆运危规: 6.1 国际海运危规: 6.1 国际空运危规: 6.1
14.4 包裹组
欧洲陆运危规: III 国际海运危规: III 国际空运危规: III
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
上述信息视为正确,但不包含所有的信息,仅作为指引使用。本文件中的信息是基于我们目前所知,就正
确的安全提示来说适用于本品。该信息不代表对此产品性质的保证。
参见发票或包装条的反面。
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
3-乙酰基吡啶是合成利塞膦酸钠的重要中间体,而利塞膦酸钠则是新一代双膦酸盐类药物的杰出代表。由美国宝洁公司开发,该药物于1998年在美国和欧洲上市,属于第三代双膦酸盐。与其他同类药相比,利塞膦酸钠抗吸收作用增强且毒副作用小,已成为目前防治骨质疏松症最主要药物之一。
含量分析按GT-10-4气相色谱法中用极性柱方法测定。
毒性GRAS(FEMA)。
使用限量FEMA(mg/kg):
- 软饮料:2.0
- 冷饮:2.0
- 糖果:3.0
- 焙烤食品:3.0
- 布丁类:2.0
- 肉类、汤类:0.05
| 添加剂中文名称 | 允许使用该种添加剂的食品中文名称 | 添加剂功能 | 最大允许使用量(g/kg) | 最大允许残留量(g/kg) |
|---|---|---|---|---|
| 3-乙酰基吡啶 | 食品 | 食用药用香料 | 用于配制香精的各香料成分不得超过在GB 2760中的最大允许使用量和最大允许残留量 |
无色至黄色液体,具有花生和坚果似的香气,类似爆玉米花的香气,并带有甜味。沸点为230℃。溶于酸类、乙醇、乙醚和水。
用途 生产方法 类别易燃液体
毒性分级高毒
急性毒性- 口服 - 大鼠 LD50: 46 微升/公斤
- 腹腔 - 小鼠 LD50: 182 毫克/公斤
可燃;加热分解释放有毒氮氧化物烟雾。
储运特性库房通风低温干燥
灭火剂上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-(1-氧化吡啶-3-基)乙酮 3-acetylpyridine N-oxide 14188-94-4 C7H7NO2 137.138 3-氧代-3-(3-吡啶基)丙腈 nicotinoylacetonitrile 30510-18-0 C8H6N2O 146.148 5-乙酰基-2-溴吡啶 1-(6-bromopyridin-3-yl)ethanone 139042-59-4 C7H6BrNO 200.035 3-吡啶甲醛 3-pyridinecarboxaldehyde 500-22-1 C6H5NO 107.112 3-乙基吡啶 3-ethylpyridine 536-78-7 C7H9N 107.155 —— 3-phenyl-1-(pyridin-3-yl)prop-2-yn-1-one 86535-76-4 C14H9NO 207.232 —— 1-phenyl-3-(pyridin-3-yl)propane-1,3-dione 10472-95-4 C14H11NO2 225.247 3-氧代-3-(3-吡啶基)丙酸乙酯 3-oxo-3-pyridin-3-yl-propionic acid ethyl ester 6283-81-4 C10H11NO3 193.202 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— pyridine-3-ylglyoxal 63464-84-6 C7H5NO2 135.122 3-(2-溴乙酰基)吡啶 3-Bromoacetylpyridine 6221-12-1 C7H6BrNO 200.035 1-(3-吡啶基)-2-丙烯-1-酮 1-(pyridin-3-yl)prop-2-en-1-one 133614-04-7 C8H7NO 133.15 3-(2-氨基乙酰基)吡啶 3-(α-aminoacetyl)pyridine 51941-15-2 C7H8N2O 136.153 2-氯-1-(吡啶-3-基)乙酮 2-chloro-1-pyridin-3-yl-ethanone 55484-11-2 C7H6ClNO 155.584 2-羟基-1-(3-吡啶)-乙酮 3-Hydroxyacetylpyridine 104501-59-9 C7H7NO2 137.138 2-甲基-1-(3-吡啶基)-1-丙酮 2-methyl-1-(pyridin-3-yl)propan-1-one 51227-29-3 C9H11NO 149.192 1-(1-氧化吡啶-3-基)乙酮 3-acetylpyridine N-oxide 14188-94-4 C7H7NO2 137.138 2-肟基-1-(3-吡啶基)-1-乙酮 2-hydroxyimino-1-(3-pyridyl)-1-ethanone 92639-84-4 C7H6N2O2 150.137 —— 2-oxo-2-(pyridin-3-yl)acetaldehyde oxime 67475-16-5 C7H6N2O2 150.137 —— 3-diazoacetylpyridine 39972-50-4 C7H5N3O 147.136 2,2-二氟-1-(3-吡啶基)乙酮 2,2-difluoro-1-(pyridin-3-yl)ethan-1-one 155557-13-4 C7H5F2NO 157.12 —— 2,2-dichloro-1-(pyridin-3-yl)ethan-1-one —— C7H5Cl2NO 190.029 1-(4-甲基吡啶-3-基)乙酮 1-(4-methyl-pyridin-3-yl)-ethanone 51227-30-6 C8H9NO 135.166 4-氧代-4-吡啶-3-基丁腈 2-cyanoethyl 3-pyridyl ketone 36740-10-0 C9H8N2O 160.175 2-羟基-5-醛基吡啶 1-(6-hydroxypyridin-3-yl)ethanone 1124-29-4 C7H7NO2 137.138 3-三氟乙酰基吡啶 2,2,2-trifluoro-1-(pyridin-3-yl)ethan-1-one 33284-21-8 C7H4F3NO 175.11 (E)-3-(二甲基氨基)-1-(吡啶-3-基)丙-2-烯-1-酮 (E)-3-(dimethylamino)-1-(pyridin-3-yl)prop-2-en-1-one 123367-26-0 C10H12N2O 176.218 1-(3-吡啶基)-3-(二甲氨基)-2-丙烯-1-酮 3-(N,N-dimethylamino)-1-(pyridin-3-yl)prop-2-en-1-one 55314-16-4 C10H12N2O 176.218 3-(二甲氨基)-1-(3-吡啶基)-2-丙烯-1-酮 3-(dimethylamino)-1-(3-pyridinyl)-2-propen-1-one 75415-01-9 C10H12N2O 176.218 3-乙基吡啶 3-ethylpyridine 536-78-7 C7H9N 107.155 3-乙酰基-2-氯吡啶 3-acetyl-2-chloropyridine 55676-21-6 C7H6ClNO 155.584 1-(3-吡啶)-1,3-丁二酮 nicotinoylacetone 3594-37-4 C9H9NO2 163.176 1,2-二吡啶-3-基乙酮 1,2-di(pyridin-3-yl)ethanone 6339-93-1 C12H10N2O 198.224 氧代(吡啶-3-基)乙酸 (3-Pyridyl)glyoxylic acid 39684-37-2 C7H5NO3 151.122 3-羟基-1-(3-吡啶基)-1-丁酮 3-hydroxy-1-(3-pyridyl)-1-butanone 100021-46-3 C9H11NO2 165.192 —— 3-ethyl-1-(pyridin-3-yl)pentan-1-one —— C12H17NO 191.273 1,3-二吡啶-3-基丙烷-1,3-二酮 1,3-bis(pyridin-3-yl)propane-1,3-dione 6327-87-3 C13H10N2O2 226.235 3-乙酰基-2(1H)-吡啶酮 1-(2-hydroxypyridin-3-yl)ethan-1-one 62838-65-7 C7H7NO2 137.138 —— (Z)-4-Oxo-4-pyridin-3-yl-but-2-enoic acid 80886-21-1 C9H7NO3 177.159 4 - (吡啶- 3 -基)-4-氧代丁酸盐酸盐 4-oxo-4-(pyridin-3-yl)butanoic acid 4192-31-8 C9H9NO3 179.175 1-丙酮,3-苯基-1-(3-吡啶基)- 3-phenyl-1-(pyridin-3-yl)propan-1-one 1802-36-4 C14H13NO 211.263 3-氧代-3-(3-吡啶基)丙酰胺 3-oxo-3-(3-pyridyl)propanamide 152171-44-3 C8H8N2O2 164.164 5-乙酰基-2-氰基吡啶 5-acetyl-2-pyridinecarbonitrile 249583-84-4 C8H6N2O 146.148 3-吡啶基苯乙酮 2-phenyl-1-(pyridin-3-yl)ethanone 14627-92-0 C13H11NO 197.236 —— 3-diethylamino-1-pyridin-3-yl-propenone 54105-05-4 C12H16N2O 204.272 —— 3,3-bis-methylsulfanyl-1-pyridin-3-yl-prop-2-en-1-one 68192-56-3 C10H11NOS2 225.335 3-(2-氨基乙基)吡啶 2-pyridin-3-ylethylamine 20173-24-4 C7H10N2 122.17 —— N,N-bis(3-pyridinoylethyl)cystamine 1265222-51-2 C20H26N4O2S2 418.584 —— (E)-1-(pyridin-3-yl)-3-(pyridin-4-yl)prop-2-en-1-one 18461-23-9 C13H10N2O 210.235 2-(4-甲基苯基)-1-(3-吡啶)-1-乙酮 1-(pyridin-3-yl)-2-(p-tolyl)ethan-1-one 40061-21-0 C14H13NO 211.263 —— (E)-3-phenyl-1-(pyridin-3-yl)prop-2-en-1-one 53940-02-6 C14H11NO 209.247 —— 2-(N-2-hydroxyethylmethyl)aminoethyl-3-pyridylketone 1219023-36-5 C11H16N2O2 208.26 (E)-3-苯基-1-吡啶-3-基-丙-2-烯-1-酮 3-phenyl-1-(pyridin-3-yl)prop-2-en-1-one 6314-59-6 C14H11NO 209.247 —— 2-(diallylamino)-1-(3-pyridyl)ethanone 1607431-84-4 C13H16N2O 216.283 —— 2-cyclohexyl-1-(pyridin-3-yl)ethanone —— C13H17NO 203.284 —— 2-(N-bishydroxyethyl)aminoethyl-3-pyridylketone 1219023-34-3 C12H18N2O3 238.287 烟酰乙酸甲酯 methyl nicotinoylacetate 54950-20-8 C9H9NO3 179.175 —— 1-pyridin-3-yl-3-p-tolyl-propenone 16795-35-0 C15H13NO 223.274 —— 1.4-Bis-<1-pyridyl-(3)-1-oxo-propen-(2)-yl-(3)>-benzol 98822-34-5 C22H16N2O2 340.381 —— (E)-1-(3-pyridinyl)-3-(3-pyridinyl)prop-2-en-1-one 145917-24-4 C13H10N2O 210.235 —— 1,3-di(pyridin-3-yl)prop-2-en-1-one 13309-07-4 C13H10N2O 210.235 —— 3-Acetyl-4-methylaminopyridine 84575-46-2 C8H10N2O 150.18 —— 1-pyridin-4-yl-3pyridin-3-yl-propane-1,3-dione —— C13H10N2O2 226.235 —— 4,4-difluoro-1-(pyridin-3-yl)butane-1,3-dione —— C9H7F2NO2 199.157 —— 1-(6-(tert-butyl)pyridin-3-yl)ethan-1-one 56029-46-0 C11H15NO 177.246 —— 2-(N-isopropylhydroxyethyl)aminoethyl-3'-pyridylketone 1219023-32-1 C13H20N2O2 236.314 —— 1-(pyridin-3-yl)-3-(tetrahydro-2H-pyran-4-yl)propan-1-one 700834-59-9 C13H17NO2 219.283 3-叔丁基吡啶 3-tert-butylpyridine 38031-78-6 C9H13N 135.209 —— 3-(Dimethylamino)-1-(3-pyridinyl)-2-buten-1-one 287494-18-2 C11H14N2O 190.245 1,1'-(2,5-吡啶二基)二乙酮 1,1'-pyridine-2,5-diyldiethanone 20857-28-7 C9H9NO2 163.176 3-二甲氨基-1-(3-吡啶基)-2-丁烯-1-酮 3-dimethylamino-1-(3-pyridyl)-2-buten-1-one 96604-06-7 C11H14N2O 190.245 烟酸 nicotinic acid 59-67-6 C6H5NO2 123.111 3-氧代-3-(3-吡啶基)丙酸乙酯 3-oxo-3-pyridin-3-yl-propionic acid ethyl ester 6283-81-4 C10H11NO3 193.202 - 1
- 2
- 3
- 4
- 5
- 6
- 7
反应信息
-
作为反应物:描述:3-乙酰基吡啶 在 sodium methylate 、 1-羟基苯并三唑 、 盐酸-N-乙基-Nˊ-(3-二甲氨基丙基)碳二亚胺 、 三乙胺 作用下, 以 甲醇 、 二甲基亚砜 为溶剂, 反应 109.6h, 生成 过氧化物酶(辣根)参考文献:名称:[EN] PROCESS FOR THE PREPARATION OF SUBSTITUTED PYRIMIDINES
[FR] PROCÉDÉ DE PRÉPARATION DE PYRIMIDINES SUBSTITUÉES摘要:这项发明涉及一种改进的制备化合物(I)及其合成中间体的方法。具体而言,该发明涉及一种改进的方法,用于制备N-(2-氨基苯基)-4-((嘧啶-2-基氨基)甲基)苯甲酰胺,该方法需要较少的步骤,高效,可用于工业规模,并得到适用于药物使用的最终产品。公开号:WO2015006875A1 -
作为产物:描述:参考文献:名称:烯烃的光催化氧化裂解和羰基立体选择性生物还原合成对映体富集的仲醇摘要:介绍 传统上,通过基于在极低温度下原位产生臭氧作为终端氧化剂的臭氧分解反应来进行烯烃的氧化裂解以产生二羰基化合物。1与臭氧化物和过氧化物形成相关的危害,特别是在下游工艺过程中、废物产生量高、需要专用设备以及在存在其他官能团的情况下缺乏选择性,提醒需要披露更安全和可持续的烯烃臭氧分解替代品。在这种情况下,使用其他氧化物质,如 KMnO 4、NaIO 4、HIO 4、OsO 4、叔丁基氢过氧化物、过硫酸氢钾…… 2种金属催化的氧化裂解(Co、Mn、Ru、Fe……),2b, 3以及涉及非血红素和血红素铁依赖性蛋白的酶转化,4为制备多种醛和酮提供了多种可能性。从2001年起,在甲氧基苯作为敏化剂的存在下,使用分子氧进行α-甲基苯乙烯的光诱导氧化是已知的。5后来,由于自然界中存在丰富的可见光,光诱导烯烃氧化裂解反应6的性能,包括金属催化(Cu、Ru、Ir…)、使用有机染料和酶的无金属转化,受到越来越DOI:10.1002/adsc.202301325
-
作为试剂:描述:1,4-二甲基-1H-吲哚 、 2-甲基恶唑-4-甲酸甲酯 在 3-乙酰基吡啶 、 copper diacetate 、 palladium diacetate 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 24.0h, 以40%的产率得到methyl 5-(1,4-dimethyl-1H-indol-3-yl)-2-methyloxazole-4-carboxylate参考文献:名称:钯催化的吡唑-4-羧酸酯与吲哚的氧化交叉偶联:一种合成嘧啶的方法。摘要:公开了一种通过在温和的条件下通过钯催化的双CH键裂解来制备结合有特权结构吲哚和唑的5-(3-吲哚基)唑的有效而直接的方法。如预期的那样,该方案为中等产率的吲哚生物碱嘧啶和WS-30581 A的合成提供了一种简便的方法。DOI:10.1039/c4ob01844c
文献信息
-
Substituted 1-benzoyl-3-cyano-pyrrolo [1,2-a] quinolines and analogs as activators of caspases and inducers of apoptosis申请人:Cai Xiong Sui公开号:US20050014759A1公开(公告)日:2005-01-20The present invention is directed to substituted 1-benzoyl-3-cyano-pyrrolo[1,2-a]quinolines and analogs thereof, represented by the general Formula I: wherein R 1 —R 8 , L, Q, dash line and Ar are defined herein. The present invention also relates to the discovery that compounds having Formula I are activators of caspases and inducers of apoptosis. Therefore, the activators of caspases and inducers of apoptosis of this invention can be used to induce cell death in a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.本发明涉及取代的1-苯甲酰基-3-氰基-吡咯并[1,2-a]喹啉及其类似物,由通用式I表示: 其中R1-R8,L,Q,虚线和Ar在此定义。本发明还涉及发现具有式I的化合物是caspase的激活剂和凋亡诱导剂。因此,本发明的caspase激活剂和凋亡诱导剂可用于诱导在各种临床病况中发生未受控制的异常细胞生长和扩散的细胞死亡。
-
Autoxidation/Aldol Tandem Reaction of 2-Oxindoles with Ketones: A Green Approach for the Synthesis of 3-Hydroxy-2-Oxindoles作者:Qing-Bao Zhang、Wen-Liang Jia、Yong-Liang Ban、Yong Zheng、Qiang Liu、Li-Zhu WuDOI:10.1002/chem.201504282日期:2016.2.18In the presence of tetrabutylammonium fluoride and molecular sieves (MS) 4 Å in DMF, an efficient autoxidation reaction of 2‐oxindoles with ketones under air at room temperature has been developed. This approach may provide a green, practical, and metal‐free protocol for a wide range of biologically important 3‐hydroxy‐3‐(2‐oxo‐alkyl)‐2‐oxindoles.
-
Certain pyrazoline derivatives with kinase inhibitory activity申请人:Adams Ruth S.公开号:US20080171754A1公开(公告)日:2008-07-17The present invention provides certain pyrazoline compounds useful as inhibitors of protein kinases. The invention also provides pharmaceutical compositions and methods of using the compositions in the treatment of various diseases.
-
Investigations into the Potential Role of Metabolites on the Anti-Leukemic Activity of Imatinib, Nilotinib and Midostaurin作者:Paul W. ManleyDOI:10.2533/chimia.2019.561日期:——
The efficacy and side-effects of drugs do not just reflect the biochemical and pharmacodynamic properties of the parent compound, but often comprise of cooperative effects between the properties of the parent and active metabolites. Metabolites of imatinib, nilotinib and midostaurin have been synthesised and evaluated in assays to compare their properties as protein kinase inhibitors with the parent drugs. The N-desmethyl-metabolite of imatinib is substantially less active than imatinib as a BCR-ABL1 kinase inhibitor, thus providing an explanation as to why patients producing high levels of this metabolite show a relatively low response rate in chronic myeloid leukaemia (CML) treatment. The hydroxymethylphenyl and N-oxide metabolites of imatinib and nilotinib are only weakly active as BCR-ABL1 inhibitors and are unlikely to play a role in the efficacy of either drug in CML. The 3-(R)-HO-metabolite of midostaurin shows appreciable accumulation following chronic drug administration and, in addition to mutant forms of FLT3, potently inhibits the PDPK1 and VEGFR2 kinases (IC50 values
药物的功效和副作用不仅仅反映了母化合物的生化和药效特性,而且通常包括母化合物和活性代谢物之间的协同效应。已经合成和评估了伊马替尼、尼洛替尼和米多斯他林的代谢物,以比较它们作为蛋白激酶抑制剂的特性与母药的区别。伊马替尼的N-去甲基代谢物作为BCR-ABL1激酶抑制剂的活性明显低于伊马替尼,这解释了为什么产生高水平该代谢物的患者在慢性髓细胞白血病(CML)治疗中显示相对较低的反应率。伊马替尼和尼洛替尼的羟甲基苯和N-氧代谢物作为BCR-ABL1抑制剂的活性很弱,不太可能在CML中发挥作用。米多斯他林的3-(R)-HO-代谢物在长期用药后显示出明显的积累,并且除了FLT3的突变形式外,还强力抑制PDPK1和VEGFR2激酶(IC50值 -
Copper-Based Intermetallic Electride Catalyst for Chemoselective Hydrogenation Reactions作者:Tian-Nan Ye、Yangfan Lu、Jiang Li、Takuya Nakao、Hongsheng Yang、Tomofumi Tada、Masaaki Kitano、Hideo HosonoDOI:10.1021/jacs.7b08252日期:2017.11.29The development of transition metal intermetallic compounds, in which active sites are incorporated in lattice frameworks, has great potential for modulating the local structure and the electronic properties of active sites, and enhancing the catalytic activity and stability. Here we report that a new copper-based intermetallic electride catalyst, LaCu0.67Si1.33, in which Cu sites activated by anionic过渡金属间化合物的开发,其中活性位点并入晶格骨架中,具有很大的潜力来调节活性位点的局部结构和电子性质,并增强催化活性和稳定性。在这里,我们报道了一种新型的铜基金属间电催化剂LaCu 0.67 Si 1.33,其中具有低功函的阴离子电子激活的Cu位原子原子地分散在晶格骨架中,并提供硝基芳烃的选择性加氢,其营业额高40倍以上频率(TOF高达5084 h –1),而不是经过深入研究的金属负载催化剂。利用同位素效应的动力学分析表明,氢键的裂解是决定速率的步骤。出乎意料的是,LaCu 0.67 Si 1.33的高载流子密度和低逸出功(LWF)特性使得能够以极低的活化能(E a = 14.8 kJ·mol –1)活化氢分子。此外,LaCu 0.67 Si 1.33的高氧亲合力可实现通过硝基优先吸附硝基芳烃表面,导致高化学选择性。本发明的有效催化剂可以进一步引发具有高活性的其他含氧官能团例如醛和酮的氢化
表征谱图
-
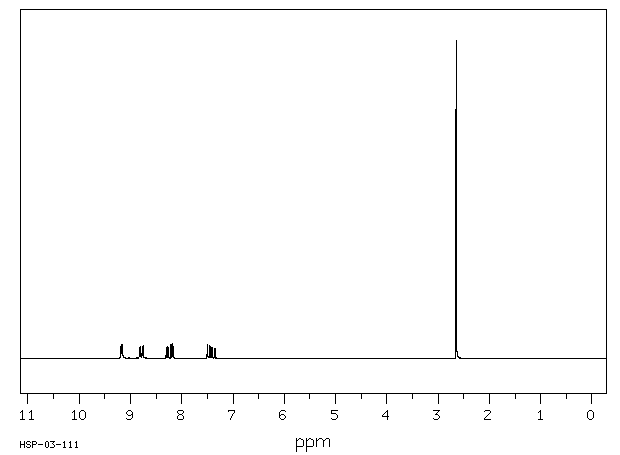
氢谱1HNMR
-
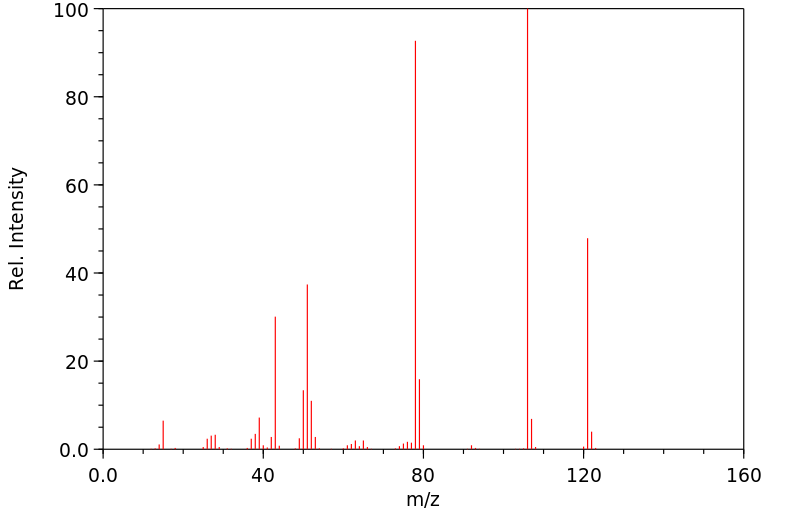
质谱MS
-
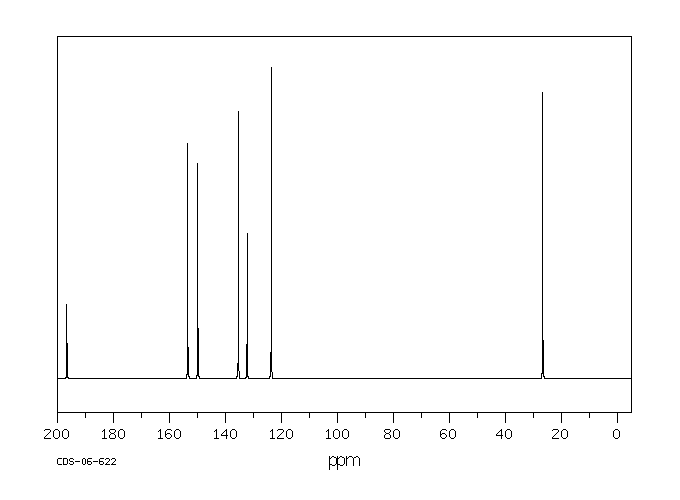
碳谱13CNMR
-
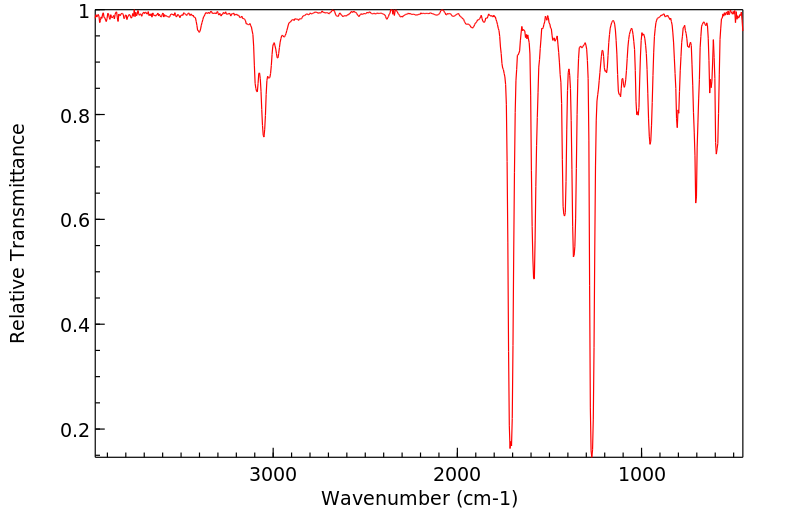
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息