1,4-丁二醇 | 110-63-4
物质功能分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:16 °C (lit.)
-
沸点:230 °C (lit.)
-
密度:1.017 g/mL at 25 °C (lit.)
-
蒸气密度:3.1 (vs air)
-
闪点:135 °C
-
介电常数:30.0(30℃)
-
LogP:-0.88 at 25℃
-
物理描述:1,4-butanediol appears as odorless colorless liquid or solid (depending upon temperature). (USCG, 1999)
-
颜色/状态:Colorless, oily liquid
-
气味:Almost odorless
-
溶解度:1000000 mg/L (at 20 °C)
-
蒸汽密度:3.1 (NTP, 1992) (Relative to Air)
-
蒸汽压力:0.0105 mm Hg at 25 °C
-
稳定性/保质期:
-
自燃温度:662 °F (350 °C)
-
分解:When heated to decomposition it emits acrid smoke and fumes.
-
粘度:84.9 mPa.s at 20 °C
-
腐蚀性:Noncorrosive
-
燃烧热:2585 kJ/mol
-
汽化热:56.5 kJ/mol at 230.5 °C
-
表面张力:44.6 mN/m at 20 °C
-
折光率:Index of refraction: 1.4460 at 20 °C
-
解离常数:pKa = 14.5
-
保留指数:912.4;922;931;903.7;915.8
计算性质
-
辛醇/水分配系数(LogP):-0.8
-
重原子数:6
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
ADMET
安全信息
-
TSCA:Yes
-
危险品标志:Xn
-
安全说明:S36
-
危险类别码:R22
-
WGK Germany:1
-
海关编码:2905399090
-
危险类别:6.1
-
RTECS号:EK0525000
-
包装等级:I; II; III
-
储存条件:储存于阴凉、通风的库房,远离火种和氧化剂。保持容器密封,并将储存地点远离水源。应配备泄漏应急处理设备及合适的收容材料。 可用软钢、铝或铜制容器密封贮存。采用铝、不锈钢、镀锌铁桶或塑料桶包装,或使用槽车按易燃有毒物品规定进行运输。由于熔点高达20℃,槽车内应安装加热管以确保安全。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 1,4-丁二醇;丁隔二醇 |
| 化学品英文名称: | 1,4-Butylene glycol;1,4-Butanediol |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 110-63-4 |
| 分子式: | C 4 H 10 O 2 |
| 分子量: | 90.12 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | |||
| 化学品名称:1,4-丁二醇;丁隔二醇 | |||
|
| 第三部分:危险性概述 |
| 危险性类别: | |
| 侵入途径: | 吸入 食入 |
| 健康危害: | 未稀释的本品对人的皮肤微有刺激作用。国外曾有人报道,7例将本品作为甘油代用品使用而引起中毒,中毒者有肾脏损害。 |
| 环境危害: | |
| 燃爆危险: | 本品可燃。 |
| 第四部分:急救措施 |
| 皮肤接触: | 脱去污染的衣着,用流动清水冲洗。 |
| 眼睛接触: | 立即翻开上下眼睑,用流动清水冲洗。就医。 |
| 吸入: | 脱离现场至空气新鲜处。就医。 |
| 食入: | 误服者给饮足量温水,催吐,就医。 |
| 第五部分:消防措施 |
| 危险特性: | 遇明火、高热可燃。与氧化剂可发生反应。若遇高热,容器内压增大,有开裂和爆炸的危险 |
| 有害燃烧产物: | 一氧化碳、二氧化碳。 |
| 灭火方法及灭火剂: | 尽可能将容器从火场移至空旷处。喷水保持火场容器冷却,直至灭火结束。处在火场中的容器若已变色或从安全泄压装置中产生声音,必须马上撤离。用水喷射逸出液体,使其稀释成不燃性混合物,并用雾状水保护消防人员。灭火剂:水、雾状水、抗溶性泡沫、干粉、二氧化碳、砂土。 |
| 消防员的个体防护: | |
| 禁止使用的灭火剂: | |
| 闪点(℃): | >110 |
| 自燃温度(℃): | 引燃温度(℃):370 |
| 爆炸下限[%(V/V)]: | 无资料 |
| 爆炸上限[%(V/V)]: | 无资料 |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自吸过滤式防毒面具(全面罩),穿一般作业工作服。尽可能切断泄漏源。防止流入下水道、排洪沟等限制性空间。小量泄漏:用砂土、干燥石灰或苏打灰混合。也可以用大量水冲洗,洗水稀释后放入废水系统。大量泄漏:构筑围堤或挖坑收容。用泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | 密闭操作,提供良好的自然通风条件。操作人员必须经过专门培训,严格遵守操作规程。远离火种、热源,工作场所严禁吸烟。使用防爆型的通风系统和设备。防止蒸气泄漏到工作场所空气中。避免与氧化剂、酸类接触。搬运时轻装轻卸,保持包装完整,防止洒漏。配备相应品种和数量的消防器材及泄漏应急处理设备。倒空的容器可能残留有害物。 |
| 储存注意事项: | 储存于阴凉、通风的库房。远离火种、热源。应与氧化剂、酸类等分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有泄漏应急处理设备和合适的收容材料。 |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中 国 MAC:未制订标准前苏联MAC:未制订标准美国TLV—TWA:未制订标准美国 |
| 监测方法: | |
| 工程控制: | 提供良好的自然通风条件。 |
| 呼吸系统防护: | 一般不需要特殊防护,高浓度接触时可佩带自给式呼吸器。 |
| 眼睛防护: | 必要时戴安全防护眼镜。 |
| 身体防护: | 穿工作服。 |
| 手防护: | 必要时戴防化学品手套。 |
| 其他防护: | 工作后,淋浴更衣。避免长期反复接触。定期体检。 |
| 第九部分:理化特性 |
| 外观与性状: | 无色、油状液体。 |
| pH: | |
| 熔点(℃): | 16 |
| 沸点(℃): | 230 |
| 相对密度(水=1): | 1.02 |
| 相对蒸气密度(空气=1): | 3.1 |
| 饱和蒸气压(kPa): | |
| 燃烧热(kJ/mol): | 601.6 |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | >110 |
| 引燃温度(℃): | 引燃温度(℃):370 |
| 爆炸上限%(V/V): | 无资料 |
| 爆炸下限%(V/V): | 无资料 |
| 分子式: | C 4 H 10 O 2 |
| 分子量: | 90.12 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 微溶于乙醚,与水混溶,溶于乙醇等。 |
| 主要用途: | 用作溶剂和增湿剂,也用于制增塑剂、药物、聚酯树脂、聚氨基甲酸酯树脂等。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强氧化剂、酰基氯、酸酐、强酸。 |
| 避免接触的条件: | |
| 聚合危害: | 不能出现 |
| 分解产物: | 一氧化碳、二氧化碳。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | 属低毒类 LD50:小鼠经口:2.2g/kg,大鼠经口:1.8g/kg LC50: |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | 处置前应参阅国家和地方有关法规。建议用焚烧法处置。 |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | |
| UN编号: | |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | |
| 运输注意事项: | 运输前应先检查包装容器是否完整、密封,运输过程中要确保容器不泄漏、不倒塌、不坠落、不损坏。严禁与氧化剂、酸类等混装混运。船运时,应与机舱、电源、火源等部位隔离。公路运输时要按规定路线行驶。 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 3 |
| MSDS修改日期: | 年月日 |
制备方法与用途
1,4-丁二醇是一种无色黏稠的液体,能与强氧化剂发生反应。它是一种重要的基本有机化工原料,主要用于生产聚对苯二甲酸二丁酯(PBT)、聚氨酯、γ-丁内酯和四氢呋喃。
化学性质1,4-丁二醇为无色油状液体,能与水混溶,并可溶解于甲醇、乙醇及丙酮中,微溶于乙醚。
用途 广泛应用1,4-丁二醇的用途十分广泛。在美国和西欧,它主要用于生产四氢呋喃(占一半以上),其次是γ-丁内酯和聚对苯二甲酸丁二醇酯。后者是一种迅速发展的工程塑料;作为增链剂和聚酯原料,1,4-丁二醇用于生产聚氨酯弹性体和软质聚氨酯泡沫塑料;制得的酯类是纤维素、聚氯乙烯、聚丙烯酸酯及聚酯的良好增塑剂。它还具有良好的吸湿性和柔韧性,可用作明胶软化剂和吸水剂,处理玻璃纸和其他未用纸。
其他用途1,4-丁二醇制备的N-甲基吡咯烷酮、N-乙烯基吡咯烷酮及其衍生物,并用于生产维生素B6、农药、除草剂以及多种工艺过程中的溶剂、增塑剂、润滑剂、增湿剂、柔软性及胶粘剂和电镀工业的光亮剂。
分析与合成化学分析用试剂,亦可作为气相色谱固定液。用于有机合成,还可作溶剂、无毒抗冻剂、食品乳化剂和吸湿剂等,广泛应用于制药和食品工业。
生产方法-
乙炔法:先以乙炔与甲醛在Cu-Bi催化剂存在下,在98kPa、80-95℃条件下反应生成1,4-丁炔二醇;再经骨架镍催化加氢成1,4-丁烯二酸盐,进一步用Ni-Cu-Mn/Al2O3催化加氢(13.7-20.6MPa、120-140℃)制成1,4-丁二醇。经过离子交换树脂除杂后蒸馏提纯得成品。
-
顺酐加氢法
-
1,4-二氯丁烯法:以1,4-二氯丁烯为原料,经过水解和加氢而得1,4-丁二醇。1,4-二氯丁烯是丁二烯生产氯丁二烯过程中的中间产物。
易燃液体
毒性分级中毒
急性毒性大鼠口服 LD50: 1525毫克/公斤;小鼠口服 LD50: 2062毫克/公斤
可燃性危险特性遇热、明火易燃,燃烧时产生刺激烟雾
储运特性库房应通风低温干燥存放,并与氧化剂分开
灭火剂干粉、泡沫、水
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 正丁醇 butan-1-ol 71-36-3 C4H10O 74.1228 丙醇 propan-1-ol 71-23-8 C3H8O 60.0959 丁二醇 butyraldehyde hydrate 25265-75-2 C4H10O2 90.1222 1,3-丙二醇 1,3-propanediol 504-63-2 C3H8O2 76.0953 四氢呋喃 tetrahydrofuran 109-99-9 C4H8O 72.1069 4-羟基丁醛 4-hydroxybutyraldehyde 25714-71-0 C4H8O2 88.1063 1,2-丁二醇 1,2-butanediol 584-03-2 C4H10O2 90.1222 1,6-己二醇 1,6-hexanediol 629-11-8 C6H14O2 118.176 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 正丁醇 butan-1-ol 71-36-3 C4H10O 74.1228 4-甲氧基-1-丁醇 4-methoxybutanol 111-32-0 C5H12O2 104.149 丙醇 propan-1-ol 71-23-8 C3H8O 60.0959 1,4-二甲氧基丁烷 1,4-dimethoxybutane 13179-96-9 C6H14O2 118.176 1,3-丁二醇 1.3-butanediol 107-88-0 C4H10O2 90.1222 丁二醇 butyraldehyde hydrate 25265-75-2 C4H10O2 90.1222 1,3-丙二醇 1,3-propanediol 504-63-2 C3H8O2 76.0953 4-碘-1-丁醇 4-iodo-butan-1-ol 3210-08-0 C4H9IO 200.019 4-溴-1-丁醇 4-Bromo-1-butanol 33036-62-3 C4H9BrO 153.019 4-氨基-1-丁醇 4-Aminobutanol 13325-10-5 C4H11NO 89.1374 4-巯基-1-丁醇 4-Mercapto-1-butanol 14970-83-3 C4H10OS 106.189 4-氯丁醇 4-Chloro-1-butanol 928-51-8 C4H9ClO 108.568 1,2-丁二醇 1,2-butanediol 584-03-2 C4H10O2 90.1222 四氢呋喃 tetrahydrofuran 109-99-9 C4H8O 72.1069 4-羟基丁醛 4-hydroxybutyraldehyde 25714-71-0 C4H8O2 88.1063 4-氟-1-丁醇 4-fluoro-1-butanol 372-93-0 C4H9FO 92.1133 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:Bennett; Heathcoat, Journal of the Chemical Society, 1929, p. 272摘要:DOI:
-
作为产物:参考文献:名称:Preparation of 1,4-butanediol摘要:1,4-丁二醇的制备过程包括在非元素Pd和/或Pt的活性组分的氢化催化剂存在下,将1,3-丁二烯二环氧转化为1,4-丁二醇。优选的氢化催化剂含有至少一个来自元素周期表Ib、VIIb或VIIIb族的元素。公开号:US05977417A1
-
作为试剂:描述:参考文献:名称:非水介质中酒精脱氢酶催化的生物还原摘要:在缺水条件下使用分离的醇脱氢酶(ADH)建立了高产的生物催化还原方法。首先,评估了无溶剂体系对“智能共底物” 1,4-丁二醇促进的ADH evo-1.1.200催化的2-丁酮还原的影响。ADH evo-1.1.200在高试剂浓度下具有出色的活性和稳定性,因此是首选的酶。但是,在无溶剂条件下,10天之内2丁酮的转化率限制为<1%。因此,评估了与水不混溶的有机溶剂,从而在MTBE和甲苯中实现了最高的转化率。选择MTBE是因为它与其他反应组分(例如2-丁酮,2-丁醇,二醇共底物和内酯副产物)相比具有不同的沸点,可以简化下游工艺。进一步,DOI:10.1002/cctc.201300841
文献信息
-
一种用于治疗肿瘤的黄酮衍生物及其应用申请人:四川福生源科技有限公司公开号:CN111620920A公开(公告)日:2020-09-04
-
[EN] POLYMERS PREPARED FROM MIXTURES OF MULTIFUNCTIONAL N-VINYLFORMAMIDE AND HYBRID REACTIVE N-VINYLFORMAMIDE CROSSLINKING MONOMER MOIETIES AND USES THEREOF<br/>[FR] POLYMÈRES PRÉPARÉS À PARTIR DE MÉLANGES DE FRAGMENTS MONOMÈRES DE RÉTICULATION N-VINYLFORMAMIDE MULTIFONCTIONNELS ET N-VINYLFORMAMIDE RÉACTIFS HYBRIDES ET LEURS UTILISATIONS申请人:ISP INVESTMENTS INC公开号:WO2011084993A1公开(公告)日:2011-07-14The present invention provides polymers resulting from polymerization of at least one reactive vinyl monomer moiety and a multifunctional N-vinylformamide crosslinking moiety; polymers resulting from polymerization of at least one reactive vinyl monomer moiety and a hybrid N-vinylformamide crosslinking moiety having at least one N-vinylformamide functionality and at least one other reactive vinyl functionality; polymers resulting from polymerization of at least one hybrid reactive N-vinylformamide monomer moiety having one N-vinylformamide functionality and at least one other reactive non-vinyl functionality and a multifunctional N-vinylformamide crosslinking moiety; and polymers resulting from polymerization of at least one hybrid reactive N-vinylformamide monomer moiety having one N-vinylformamide functionality and at least one other reactive non-vinyl functionality and a hybrid N-vinylformamide crosslinking moiety having at least one N-vinylformamide functionality and at least one other reactive vinyl functionality. The invention further provides a wide variety of compositions comprising the novel crosslinked polymers.本发明提供了由至少一种反应性乙烯单体基团和多功能N-乙烯基甲酰胺交联基团聚合而成的聚合物;由至少一种反应性乙烯单体基团和具有至少一种N-乙烯基甲酰胺功能性和至少一种其他反应性乙烯功能性的混合N-乙烯基甲酰胺交联基团聚合而成的聚合物;由至少一种具有一种N-乙烯基甲酰胺功能性和至少一种其他反应性非乙烯功能性的混合反应性N-乙烯基甲酰胺单体基团和多功能N-乙烯基甲酰胺交联基团聚合而成的聚合物;以及由至少一种具有一种N-乙烯基甲酰胺功能性和至少一种其他反应性非乙烯功能性的混合反应性N-乙烯基甲酰胺单体基团和具有至少一种N-乙烯基甲酰胺功能性和至少一种其他反应性乙烯功能性的混合N-乙烃基甲酰胺交联基团聚合而成的聚合物。该发明还提供了包含新型交联聚合物的各种组合物。
-
[EN] MERTK DEGRADERS AND USES THEREOF<br/>[FR] AGENTS DE DÉGRADATION DE MERTK ET LEURS UTILISATIONS申请人:KYMERA THERAPEUTICS INC公开号:WO2020010210A1公开(公告)日:2020-01-09The present invention provides compounds, compositions thereof, and methods of using the same.本发明提供了化合物、其组合物以及使用相同的方法。
-
BRM TARGETING COMPOUNDS AND ASSOCIATED METHODS OF USE申请人:Arvinas Operations, Inc.公开号:US20190300521A1公开(公告)日:2019-10-03The present disclosure relates to bifunctional compounds, which find utility as modulators of SMARCA2 or BRM (target protein). In particular, the present disclosure is directed to bifunctional compounds, which contain on one end a ligand that binds to the Von Hippel-Lindau E3 ubiquitin ligase, and on the other end a moiety which binds the target protein, such that the target protein is placed in proximity to the ubiquitin ligase to effect degradation (and inhibition) of target protein. The present disclosure exhibits a broad range of pharmacological activities associated with degradation/inhibition of target protein. Diseases or disorders that result from aggregation or accumulation of the target protein are treated or prevented with compounds and compositions of the present disclosure.本公开涉及双功能化合物,其作为SMARCA2或BRM(靶蛋白)的调节剂具有实用性。具体而言,本公开涉及包含一端结合Von Hippel-Lindau E3泛素连接酶的配体,另一端结合靶蛋白的双功能化合物,使得靶蛋白与泛素连接酶靠近以实现靶蛋白的降解(和抑制)。本公开展示了与靶蛋白降解/抑制相关的广泛药理活性。本公开的化合物和组合物用于治疗或预防由靶蛋白聚集或积累导致的疾病或紊乱。
-
TAU-PROTEIN TARGETING PROTACS AND ASSOCIATED METHODS OF USE申请人:Arvinas, Inc.公开号:US20180125821A1公开(公告)日:2018-05-10The present disclosure relates to bifunctional compounds, which find utility as modulators of tau protein. In particular, the present disclosure is directed to bifunctional compounds, which contain on one end a VHL or cereblon ligand which binds to the E3 ubiquitin ligase and on the other end a moiety which binds tau protein, such that tau protein is placed in proximity to the ubiquitin ligase to effect degradation (and inhibition) of tau. The present disclosure exhibits a broad range of pharmacological activities associated with degradation/inhibition of tau protein. Diseases or disorders that result from aggregation or accumulation of tau protein are treated or prevented with compounds and compositions of the present disclosure.本公开涉及双功能化合物,其作为tau蛋白的调节剂具有实用性。具体而言,本公开涉及含有一端结合到E3泛素连接酶的VHL或cereblon配体,另一端结合到tau蛋白的双功能化合物,使得tau蛋白与泛素连接酶靠近,以实现tau蛋白的降解(和抑制)。本公开展示了与tau蛋白降解/抑制相关的广泛药理活性。本公开的化合物和组合物用于治疗或预防由tau蛋白聚集或积累导致的疾病或紊乱。
表征谱图
-
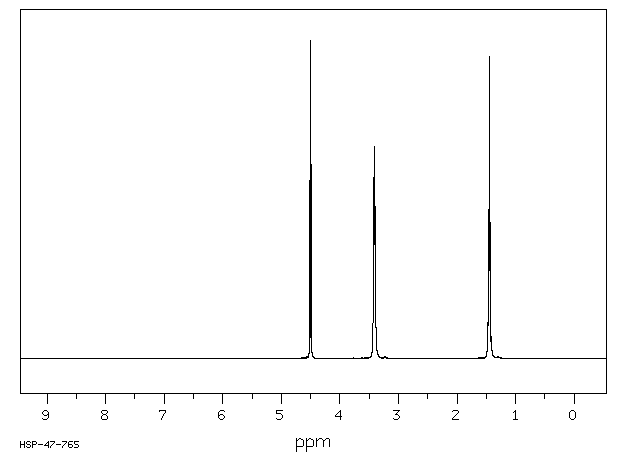
氢谱1HNMR
-
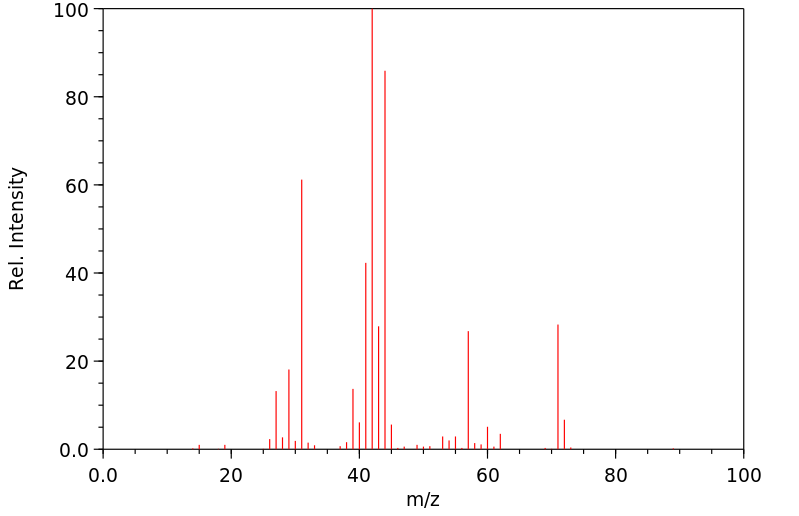
质谱MS
-
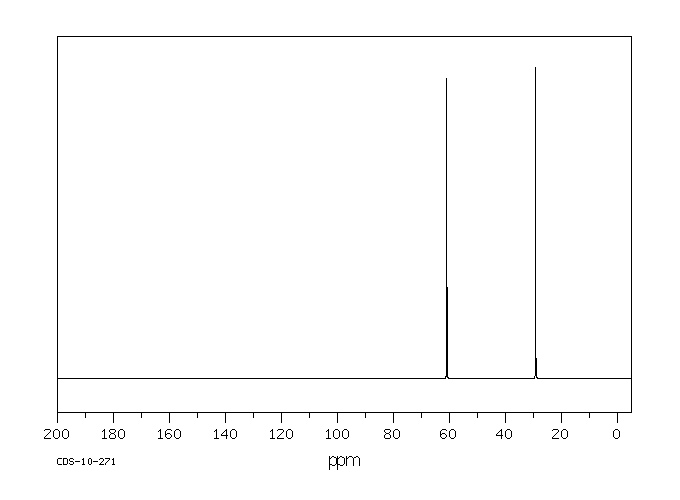
碳谱13CNMR
-
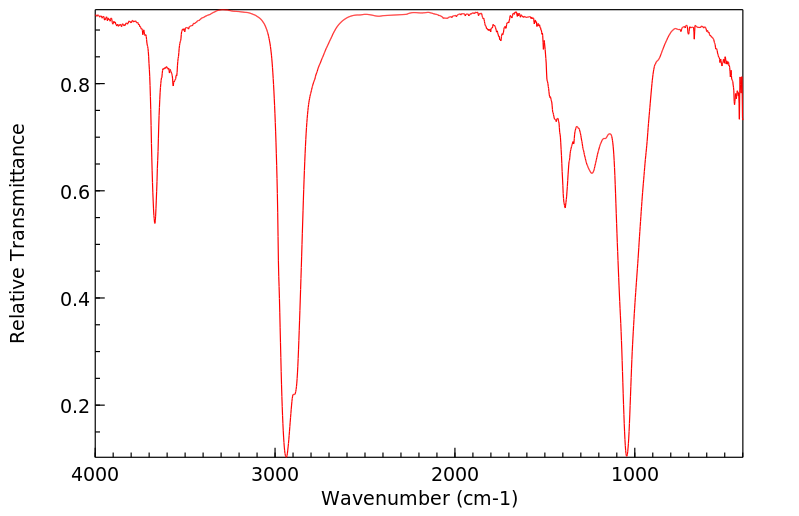
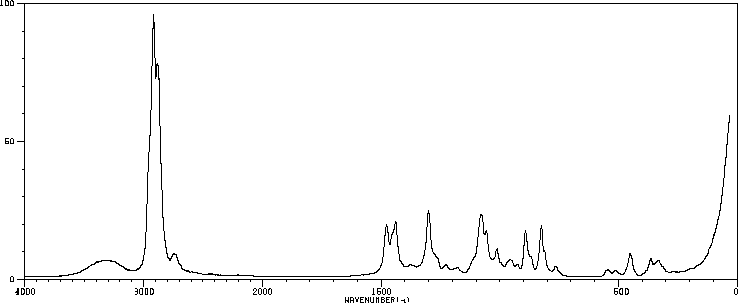
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息