辛醇 | 111-87-5
中文名称
辛醇
中文别名
伯辛醇;正辛烷醇;正辛醇;亚羊脂醇;n-辛醇;1-辛醇;1--辛醇;8醇;C8醇
英文名称
octanol
英文别名
1-Octanol;n-octanol;octan-1-ol;1‐octanol
CAS
111-87-5
化学式
C8H18O
mdl
MFCD00002988
分子量
130.23
InChiKey
KBPLFHHGFOOTCA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:−15 °C(lit.)
-
沸点:196 °C(lit.)
-
密度:0.827 g/mL at 25 °C(lit.)
-
蒸气密度:4.5 (vs air)
-
闪点:178 °F
-
溶解度:在23°C部分溶于水,溶解度为107g/L
-
最大波长(λmax):λ: 215 nm Amax: 1.00λ: 225 nm Amax: 0.50λ: 235 nm Amax: 0.20λ: 250 nm Amax: 0.05λ: 300-400 nm Amax: 0.01
-
介电常数:10.3(20℃)
-
LogP:3.5 at 23℃
-
物理描述:Octanol appears as a clear colorless liquid with a penetrating aromatic odor. Insoluble in water and floats on water. Vapors heavier than air. Vapors may irritate the eyes, nose, and respiratory system.
-
颜色/状态:Colorless liquid
-
气味:Fresh orange rose odor
-
味道:OILY, SWEET, SLIGHTLY HERBACEOUS TASTE
-
蒸汽密度:4.5 (AIR= 1)
-
蒸汽压力:7.94X10-2 mm Hg at 25 °C (est)
-
亨利常数:2.45e-05 atm-m3/mole
-
大气OH速率常数:1.44e-11 cm3/molecule*sec
-
自燃温度:253 °C
-
粘度:7.288 mPa.s at 25 °C
-
汽化热:70.98 kJ/mol at 25 °C
-
表面张力:27.53 DYNES/CM AT 20 °C (IN CONTACT WITH AIR)
-
气味阈值:The odor and taste threshold for 1-octanol was reported at about 24 ppm.
-
折光率:Index of refraction = 1.4205 at 20 °C/D
-
保留指数:1054;1052;1050;1068;1057;1054;1051;1053.2;1051;1073;1054;1054;1070;1064;1065;1065;1067;1056;1055.4;1054;1052;1061;1052;1061.7;1061.7;1058;1060;1051;1069;1054;1071;1059;1052;1053;1052;1058;1060;1057;1059;1073;1061;1051.43;1051.79;1052.35;1052.54;1059.7;1059.9;1060.5;1060.9;1059.7;1059.9;1060.5;1060.9;1052.99;1055.41;1053.9;1062;1053;1060;1034.8;1042.3;1053;1059;1060;1060;1061;1051.7;1051;1082;1061;1052;1053;1053;1053;1054;1054;1054;1055;1057;1060;1061;1066;1063;1049;1042;1049;1050;1057;1061;1062;1060;1067;1070;1061;1063;1055;1060;1093;1093;1058;1061;1065;1058;1060;1064;1057;1061;1052;1063;1055;1056;1061;1052;1054;1054;1052;1077;1049;1064;1057;1051;1051;1052;1053;1052;1045;1053;1054;1064;1073;1051;1068;1039;1048;1055;1064;1059;1039;1052;1061;1057;1055.9;1061;1070;1061;1052;1048;1052;1053;1052;1050;1053;1053;1056.8;1056;1057;1052;1055;1084;1069;1053;1050.7;1048;1050;1074;1074;1056;1056;1064.3;1059;1056;1061;1052;1053;1060;1051;1061;1054;1054;1053;1053;1054;1054;1053;1054;1061;1061;1058;1057;1071;1072;1078;1095;1056;1057;1050;1056;1052;1058;1059;1057;1083;1061;1057
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:9
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
ADMET
代谢
主要脂肪醇在体内发生两种一般反应,即氧化成羧酸和与葡萄糖醛酸直接结合。第一种反应通过中间形成醛进行,从醛中产生的羧酸可以被完全氧化成二氧化碳或者作为二氧化碳排出,或者与葡萄糖醛酸结合成酯葡萄糖苷酸。一种醇进行第二种反应的程度,即直接与醚葡萄糖苷酸结合,似乎取决于第一种反应的速度。那些迅速氧化的醇,除非以高剂量给予,否则只会形成很少的醚葡萄糖苷酸。
The primary aliphatic alcohols undergo two general reactions in vivo, namely oxidation to carboxylic acids and direct conjugation with glucuronic acid. The first reaction proceeds with the intermediate formation of an aldehyde, and the carboxylic acid from the aldehyde may be either oxidized completely to carbon dioxide or excreted as such or combined with glucuronic acid as an ester glucuronide. The extent to which an alcohol undergoes the second reaction, i.e. direct conjugation to an ether glucuronide, appears to depend upon the speed of the first reaction. Alcohols which are rapidly oxidized form very little ether glucuronide unless given in high doses.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Fischer 344大鼠通过灌胃给予正辛烷后,尿液中代谢产物包括2-辛醇、3-辛醇、5-氧代己酸和6-氧代庚酸。动物的性别影响了代谢产物形成的相对量。分析是通过气液相色谱(GC)和气液相色谱/质谱(GC/MS)进行的。这是首次报告在烃类氧化代谢中发现酮酸。尽管2,2,4-三甲基戊烷(异辛烷)同分异构体会在雄性大鼠中引起肾脏病变,但正辛烷剂量并未发现肾脏损伤。
The urinary metabolites of n-octane in Fischer 344 rats given the hydrocarbon by gavage included 2-octanol, 3-octanol, 5-oxohexanoic acid, and 6-oxoheptanoic acid. The sex of the animals influenced the relative amounts of metabolites formed. Analyses were performed by gas-liquid chromatography (GC) and gas-liquid chromatography/mass spectrometry (GC/MS). This is the first reported finding of keto acids in hydrocarbon oxidative metabolism. No kidney damage was found as a result of n-octane dosing although the 2,2,4-trimethylpentane (iso-octane) isomer does cause kidney lesions in male rats. /n-Octane/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
识别和使用:1-辛醇是一种无色液体,用于确定分配系数、香水、化妆品、有机合成、制造高沸点酯类的溶剂、消泡剂,以及作为调味剂。1-辛醇在美国注册为杀虫剂使用,但批准的杀虫剂用途可能会定期更改,因此必须咨询联邦、州和地方当局以获取当前批准的用途。它曾被用作实验性药物来治疗患者的震颤。人类暴露和毒性:在人类贴片测试中,2%矿脂中的1-辛醇既不是皮肤刺激物也不是皮肤致敏物。辛醇曾导致角膜上皮的暂时性损伤,48小时内恢复。暴露于1-辛醇的常见症状包括:中枢神经系统:头痛、肌肉无力、眩晕、共济失调、混乱、精神错乱、昏迷。胃肠道:恶心、呕吐、腹泻(排泄物中的酒精气味)。皮肤、眼睛、喉咙受到蒸汽或液体的刺激,伴有咳嗽和呼吸困难。1-辛醇的讨厌气味可能会掩盖感官刺激,阻止对化学物质敏感度增强的受试者在执行高要求任务时集中注意力。动物研究:1-辛醇对兔子的皮肤略有刺激,根据欧盟标准,被认为是一种眼睛刺激物。正辛醇(0.55 g/kg)导致体温最大程度下降。在小鼠肺部腺瘤研究中,没有发现肿瘤的证据,其中小鼠每周三次腹腔注射100和500 mg/kg的1-辛醇,持续8周。研究表明,丘脑中的T型钙通道(T通道)是全身麻醉药物的细胞靶标。该研究记录了来自年轻大鼠脑片中的网状丘脑核(nRT)神经元的T电流及其潜在的低阈值钙尖峰,并探讨了它们被一种麻醉酒精1-辛醇调节的机制。1-辛醇在亚麻醉浓度下抑制了原生T电流,IC(50)约为4 uM。原生和重组T电流的抑制伴随着稳态失活时的超极化偏移,表明1-辛醇稳定了通道的非活动状态。在大鼠的发展研究中,没有观察到与处理相关的效果,包括怀孕雌性的吸收频率、胎儿体重或骨骼/内脏畸形。在Ames沙门氏菌试验中,1-辛醇在浓度范围为4至2500 ug/板,有或没有代谢激活的情况下,对菌株TA 98、TA 100、TA 1535、TA 1537和TA 1538呈阴性反应。
IDENTIFICATION AND USE: 1-Octanol is a colorless liquid used in the determination of partition coefficients, perfumery, cosmetics, organic synthesis, solvent manufacture of high-boiling esters, antifoaming agent, and as a flavoring agent. 1-octanol is registered for pesticide use in the USA but approved pesticide uses may change periodically and so federal, state and local authorities must be consulted for currently approved uses. It has been used as experimental medication to treat tremor in patients. HUMAN EXPOSURE AND TOXICITY: In a human patch test, 1-octanol in 2% petrolatum was neither a skin irritant nor a skin sensitizer. Octanol has caused transient injury of corneal epithelium, with recovery in 48 hr. Common signs of exposure to 1-octanol are CNS: headache, muscle weakness, giddiness, ataxia, confusion, delirium, coma. Gastrointestinal: nausea, vomiting, diarrhea (odor of the alcohol in excreta). Irritation of skin, eyes, throat from vapor or liquid with cough and dyspnea. The annoying odor of 1-octanol may mask sensory irritation and prevent subjects with enhanced chemical sensitivity from concentrating on performance in a demanding task. ANIMAL STUDIES: 1-Octanol was slightly irritating to the skin of rabbits and is considered an eye irritant using the EU criteria. n-Octanol (0.55 g/kg) produced the largest decrease in body temperature. No evidence of tumors in the lung adenoma study in which mice were injected intraperitoneally with 100 and 500 mg/kg 1-octanol three times a week for 8 weeks. Studies indicate that T-type calcium channels (T-channels) in the thalamus are cellular targets for general anesthetics. The study recorded T-currents and underlying low-threshold calcium spikes from neurons of nucleus reticularis thalami (nRT) in brain slices from young rats and investigated the mechanisms of their modulation by an anesthetic alcohol, 1-octanol. 1-Octanol inhibited native T-currents at subanesthetic concentrations with an IC(50) of approximately 4 uM. Inhibition of both native and recombinant T-currents was accompanied by a hyperpolarizing shift in steady-state inactivation, indicating that 1-octanol stabilized inactive states of the channel. In developmental studies in rats no treatment-related effects were observed in pregnant females, including frequency of resorptions, fetal weights, or skeletal/visceral malformations. 1-Octanol was negative in an Ames Salmonella assay with strains Ta 98, TA 100, TA 1535, TA 1537, and TA 1538 at concentrations ranging from 4 to 2500 ug/plate with and without metabolic activation.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
这种物质可以通过吸入和摄入被身体吸收。
The substance can be absorbed into the body by inhalation and by ingestion.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
咳嗽。喉咙痛。
Cough. Sore throat.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
干燥的皮肤。
Dry skin.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
红斑。疼痛。
Redness. Pain.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
吸收、分配和排泄
体外人皮肤(表皮)的经皮流量报告为0.008毫克/平方厘米/小时,这表明渗透率较低。
In vitro dermal flux in human skin (epidermis) was reported as 0.008 mg/sq cm/hr, suggesting a low rate of penetration.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
TSCA:Yes
-
危险等级:9
-
危险品标志:Xi
-
安全说明:S26,S36/37,S37/39
-
危险类别码:R36/38
-
WGK Germany:1
-
海关编码:2905161000
-
危险品运输编号:3082
-
危险类别:9
-
RTECS号:RH6550000
-
包装等级:III
-
危险标志:GHS07
-
危险性描述:H319,H412
-
危险性防范说明:P273,P280,P305 + P351 + P338,P337 + P313
-
储存条件:1. 储存于阴凉、通风的库房。远离火种和热源。 2. 应与氧化剂、酸类及食用化学品分开存放,切忌混储。 3. 配备相应品种和数量的消防器材。储区应备有泄漏应急处理设备和合适的收容材料。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 正辛醇;1-辛醇 |
| 化学品英文名称: | Octanol;Capryl alcohol |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 111-87-5 |
| 分子式: | C 8 H 18 O |
| 分子量: | 130.23 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | ||||||||
| 化学品名称:正辛醇;1-辛醇 | ||||||||
| ||||||||
| 第三部分:危险性概述 |
| 危险性类别: | |
| 侵入途径: | 吸入 食入 经皮吸收 |
| 健康危害: | 本品对眼睛、皮肤、粘膜和上呼吸道有刺激作用。 |
| 环境危害: | |
| 燃爆危险: | 本品可燃,具刺激性。 |
| 第四部分:急救措施 |
| 皮肤接触: | 脱去污染的衣着,用肥皂水及清水彻底冲洗。 |
| 眼睛接触: | 立即翻开上下眼睑,用流动清水冲洗15分钟。就医。 |
| 吸入: | 脱离现场至空气新鲜处。呼吸困难时给输氧。呼吸停止时,立即进行人工呼吸。就医。 |
| 食入: | 误服者用水漱口,饮是量温水,催吐。就医。 |
| 第五部分:消防措施 |
| 危险特性: | 遇高热、明火或与氧化剂接触,有引起燃烧的危险。 |
| 有害燃烧产物: | 一氧化碳、二氧化碳。 |
| 灭火方法及灭火剂: | 雾状水、泡沫、二氧化碳、干粉、砂土。 |
| 消防员的个体防护: | 消防人员须佩戴防毒面具、穿全身消防服 |
| 禁止使用的灭火剂: | |
| 闪点(℃): | 81 |
| 自燃温度(℃): | 无资料 |
| 爆炸下限[%(V/V)]: | 无资料 |
| 爆炸上限[%(V/V)]: | 无资料 |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 切断火源。应急处理人员戴自给式呼吸器,穿一般消防防护服。在确保安全情况下堵漏。用砂土或其它不燃性吸附剂混合吸收,然后收集运至废物处理场所。也可以用不燃性分散剂制成的乳液刷洗,经稀释的洗液放入废水系统。如大量泄漏,利用围堤收容,然后收集、转移、回收或无害处理后废弃。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | 密闭操作,全面通风。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴自吸过滤式防毒面具(半面罩),戴化学安全防护眼镜,穿防毒物渗透工作服,戴橡胶手套。远离火种、热源,工作场所严禁吸烟。使用防爆型的通风系统和设备。防止蒸气泄漏到工作场所空气中。避免与氧化剂、酸类接触。搬运时要轻装轻卸,防止包装及容器损坏。配备相应品种和数量的消防器材及泄漏应急处理设备。倒空的容器可能残留有害物。 |
| 储存注意事项: | 储存于阴凉、通风的库房。远离火种、热源。应与氧化剂、酸类、食用化学品分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有泄漏应急处理设备和合适的收容材料。 |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中 国 MAC:未制订标准前苏联MAC:10mg/m3 美国TLV—TWA:未制订标准 |
| 监测方法: | |
| 工程控制: | 生产过程密闭,全面通风。 |
| 呼吸系统防护: | 空气中浓度较高时,应该佩戴防毒面具。 |
| 眼睛防护: | 一般不需特殊防护,但建议特殊情况下,戴化学安全防护眼镜。 |
| 身体防护: | 穿工作服。 |
| 手防护: | 必要时戴防护手套。 |
| 其他防护: | 工作现场严禁吸烟。保持良好的卫生习惯。 |
| 第九部分:理化特性 |
| 外观与性状: | 无色液体,有刺激性气味。 |
| pH: | |
| 熔点(℃): | -16.7 |
| 沸点(℃): | 196 |
| 相对密度(水=1): | 0.83(20℃) |
| 相对蒸气密度(空气=1): | 4.48 |
| 饱和蒸气压(kPa): | 0.13(54℃) |
| 燃烧热(kJ/mol): | 5275.2 |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | 81 |
| 引燃温度(℃): | 无资料 |
| 爆炸上限%(V/V): | 无资料 |
| 爆炸下限%(V/V): | 无资料 |
| 分子式: | C 8 H 18 O |
| 分子量: | 130.23 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 不溶于水,溶于乙醇、乙醚、氯仿。 |
| 主要用途: | 用作溶剂、增塑剂、防冻剂,还用于制作香精和化妆品。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强氧化剂、酸类、酰基氯。 |
| 避免接触的条件: | |
| 聚合危害: | 不能出现 |
| 分解产物: | 一氧化碳、二氧化碳。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | 属低毒类 LD50:1790mg/kg(小鼠经口);>3200mg/kg(大鼠经口) LC50: |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | 处置前应参阅国家和地方有关法规。建议用焚烧法处置。 |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | |
| UN编号: | |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | |
| 运输注意事项: | 运输前应先检查包装容器是否完整、密封,运输过程中要确保容器不泄漏、不倒塌、不坠落、不损坏。严禁与氧化剂、酸类、食用化学品等混装混运。运输车船必须彻底清洗、消毒,否则不得装运其它物品。船运时,配装位置应远离卧室、厨房,并与机舱、电源、火源等部位隔离。公路运输时要按规定路线行驶。 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | 化学危险物品安全管理条例 (1987年2月17日国务院发布),化学危险物品安全管理条例实施细则 (化劳发[1992] 677号),工作场所安全使用化学品规定 ([1996]劳部发423号)等法规,针对化学危险品的安全使用、生产、储存、运输、装卸等方面均作了相应规定。 |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 3 |
| MSDS修改日期: | 年月日 |
制备方法与用途
用途
辛醇主要用于生产增塑剂、萃取剂和稳定剂,同时也可用作溶剂和香料的中间体。在增塑剂领域,常指2-乙基乙醇(正辛醇),是一种重要的原料。辛醇还可以用作香料,用于调合玫瑰、百合等花香香精,并作为皂用香料。此外,它被允许使用于食品香料中,主要用于制备椰子、菠萝、桃子、巧克力和柑橘类香精。辛醇也可用作其他化工领域的原料,如辛醛、辛酸及其酯的原料,溶剂、消泡剂和润滑油添加剂等。在实验室中,辛醇还可用于有机合成、气相色谱分析标准等。
食品添加剂最大允许使用量与残留量 应用辛醇,又称正辛醇,是一种无色透明的油状液体,具有强烈的油脂气味和柑橘香味。它不溶于水,但能与乙醇、乙醚和氯仿混溶。主要应用于制备邻苯二甲酸二辛酯、对苯二甲酸二辛酯、丙烯酸辛酯、壬二酸二辛酯、癸二酸二辛酯等化合物。此外,它还可用作溶剂、增塑剂、防冻剂、润滑剂、萃取剂、分散剂、稳定剂和香料等。广泛应用于成品油、塑料、涂料、印染、食品加工、化妆品等领域。
制备方法工业制法主要有丙烯羰基合成法和乙醛醇醛缩合法三种。全球范围内,90%的丁辛醇生产装置采用了DAVY工艺技术或联碳低压生产工艺。但目前大多数辛醇装置多为丁辛醇联产装置,主要工业生产方法包括乙醛缩合、齐格勒法和羰基合成法,其中低压羰基合成法是当今世界最主要的丁辛醇生产技术,包括戴维合成法、三菱化成合成法和巴斯夫合成法。我国丁辛醇生产技术主要采用戴维合成法或由此演变发展而来。
化学性质- 物理性质:无色油状液体,沸点195℃,相对密度0.822-0.830,折射率1.428-1.431,闪点82.5℃。溶于乙醇(5体积的50%)或60%乙醇及油类中,酸值<1.0。
- 香气特征:干甜而尖锐的脂蜡香气,带柑橘、橙皮和玫瑰的气息,类似防风根的膏样底香,留香不长。具有油脂果香,甜且略带草香味。
辛醇在苦橙、柚、甜橙、绿茶、紫罗兰叶等精油中以游离态或酯类形式存在。工业生产时,可通过将辛醛还原或利用椰子油中存在的辛酸制备。也可采用庚烯-1为原料的羰基合成法制得。
类别- 易燃液体
-
毒性分级:中毒
- 急性毒性(口服)- 小鼠LD50: 1790毫克/公斤
- 刺激数据(皮肤)- 兔子500毫克/24小时 轻度
- 可燃性危险特性:遇明火、高温、强氧化剂可燃;燃烧排放刺激烟雾
- 库房通风,低温干燥
- 泡沫、二氧化碳、雾状水、砂土
短时间暴露极限(STEL)10毫克/立方米
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,8-辛二醇 1,8-Octanediol 629-41-4 C8H18O2 146.23 癸醇 1-Decanol 112-30-1 C10H22O 158.284 正己醇 hexan-1-ol 111-27-3 C6H14O 102.177 1-甲氧基-辛烷 1-methoxyoctane 929-56-6 C9H20O 144.257 辛基过氧化物 octyl hydroperoxide 7530-07-6 C8H18O2 146.23 8-氯-1-辛醇 8-chlorooctan-1-ol 23144-52-7 C8H17ClO 164.675 1,2-辛二醇 1,2-octandiol 1117-86-8 C8H18O2 146.23 二辛醚 di-n-octyl ether 629-82-3 C16H34O 242.445 1-乙氧基辛烷 ethyl octyl ether 929-61-3 C10H22O 158.284 —— n-octyl ethynyl ether 101537-26-2 C10H18O 154.252 辛酸 Octanoic acid 124-07-2 C8H16O2 144.214 甲酸辛酯 octyl formate 112-32-3 C9H18O2 158.241 氯甲基辛基醚 octyl chloromethyl ether 24566-90-3 C9H19ClO 178.702 壬酸 nonanoic acid 112-05-0 C9H18O2 158.241 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,8-辛二醇 1,8-Octanediol 629-41-4 C8H18O2 146.23 癸醇 1-Decanol 112-30-1 C10H22O 158.284 1,16-十六烷二醇 1,16-hexadecanediol 7735-42-4 C16H34O2 258.445 1-壬醇 nonyl alcohol 143-08-8 C9H20O 144.257 正庚醇 1-Heptanol 111-70-6 C7H16O 116.203 辛烷-1,7-二醇 octane-1,7-diol 13175-32-1 C8H18O2 146.23 1-甲氧基-辛烷 1-methoxyoctane 929-56-6 C9H20O 144.257 辛基过氧化物 octyl hydroperoxide 7530-07-6 C8H18O2 146.23 —— n-O-deuterooctanol 10330-30-0 C8H18O 131.222 次氯酸辛酯 hypochlorous acid octyl ester 203789-47-3 C8H17ClO 164.675 —— O-octyl-hydroxylamine 5663-93-4 C8H19NO 145.245 戊醇 pentan-1-ol 71-41-0 C5H12O 88.1497 4-三癸醇 tridencan-4-ol 26215-92-9 C13H28O 200.365 —— hexadecan-7-ol 591-72-0 C16H34O 242.445 3-正癸醇 decan-3-ol 1565-81-7 C10H22O 158.284 十五烷-8-醇 pentadecan-8-ol 1653-35-6 C15H32O 228.418 2-甲基-1-辛醇 2-methyloctan-1-ol 818-81-5 C9H20O 144.257 1,6-辛二醇 Octan-1,6-diol 4066-76-6 C8H18O2 146.23 1,5-辛二醇 1,5-octanediol 2736-67-6 C8H18O2 146.23 二辛醚 di-n-octyl ether 629-82-3 C16H34O 242.445 1-乙氧基辛烷 ethyl octyl ether 929-61-3 C10H22O 158.284 —— 1-butoxyoctane 53839-23-9 C12H26O 186.338 —— decyl-octyl ether 17088-93-6 C18H38O 270.499 正己基正辛醚 1-(hexyloxy)octane 17071-54-4 C14H30O 214.392 —— 1-(heptyloxy)octane 32357-84-9 C15H32O 228.418 1,4-辛烷二醇 1,4-octanediol 51916-47-3 C8H18O2 146.23 1-(乙烯氧基)辛烷 n-octyl vinyl ether 929-62-4 C10H20O 156.268 溴甲基辛基醚 bromomethyl octyl ether 96384-68-8 C9H19BrO 223.153 壬酸 nonanoic acid 112-05-0 C9H18O2 158.241 甲酸辛酯 octyl formate 112-32-3 C9H18O2 158.241 辛酸 Octanoic acid 124-07-2 C8H16O2 144.214 8-羟基辛酸 8-hydroxycaprylic acid 764-89-6 C8H16O3 160.213 氯甲基辛基醚 octyl chloromethyl ether 24566-90-3 C9H19ClO 178.702 月桂酸 laurate 143-07-7 C12H24O2 200.321 —— n-octyl ethynyl ether 101537-26-2 C10H18O 154.252 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:参考文献:名称:Ni-Catalyzed Carboxylation of Unactivated Primary Alkyl Bromides and Sulfonates with CO2摘要:A Ni-catalyzed carboxylation of unactivated primary alkyl bromides and sulfonates with CO2 at atmospheric pressure is described. The method is characterized by its mild conditions and remarkably wide scope without the need for air- or moisture-sensitive reagents, which make it a user-friendly and operationally simple protocol en route to carboxylic acids.DOI:10.1021/ja5064586
-
作为产物:参考文献:名称:铱在酸性条件下使用氢促进末端环氧化物转化为伯醇†摘要:提出了将末端环氧化物转化为伯醇的策略。该反应使用氢作为唯一的化学计量试剂,并在酸性条件下被铱预催化剂促进。对于烷基和芳基取代的末端环氧化物,都观察到在内部醇上形成末端醇的选择性,分离产率分别高达50%和72%。DOI:10.1039/c9cy00791a
-
作为试剂:描述:zirconium(IV) oxychloride 、 lead(II) acetate trihydrate 、 barium(II) acetate 在 辛醇 、 sodium hydroxide 作用下, 以 环己烷 、 水 为溶剂, 反应 34.0h, 生成 barium lead zirconate参考文献:名称:Ba 1-x Pb x ZrO 3 (0.05 ≤ x ≤ 0.20) 纳米颗粒的微乳液合成、结构表征和介电性能摘要:摘要 采用反相微乳液法成功制备了Ba 1-x Pb x ZrO 3 (x = 0.05、0.10、0.15和0.20)单相纳米粒子。X 射线衍射研究证实了 (Ba,Pb)ZrO 3 单相固溶体中的晶格收缩。TEM 研究表明,形成了粒径在 42-70 nm 范围内的多分散颗粒(球形、立方体和六边形)。发现所制备的纳米颗粒的表面积在 64-157 m 2 g -1 的范围内。发现介电常数增加 (27.2–89.5) 而发现介电损耗随着 Pb 2+ 离子浓度的增加 (0.05–0.20) 而减少 (0.07–0.002),表明合成纳米粒子的有效介电特性。合适的孔隙形态和高温稳定性使该材料成为开发电容式传感器的重要介质。观察到传感器的电容随着湿度的增加而增加,因为纳米颗粒可能适用于蒸汽感测应用。DOI:10.1016/j.materresbull.2017.01.044
文献信息
-
[EN] COMPOUNDS FOR USE AS PROTON CHANNELS AND METHODS THEREOF<br/>[FR] COMPOSÉS DESTINÉS À ÊTRE UTILISÉS EN TANT QUE CANAUX DE PROTONS ET PROCÉDÉS ASSOCIÉS申请人:AGENCY SCIENCE TECH & RES公开号:WO2020159441A1公开(公告)日:2020-08-06The present disclosure relates generally to compounds or a salt, solvate, stereoisomer and prodrug thereof for forming synthetic membrane channels. The present disclosure also relates to methods of synthesizing the compounds, methods of forming the synthetic membrane channels and methods of use thereof. In particular, the synthetic membrane channels are synthetic proton channels in a lipid membrane.本公开涉及一般用于形成合成膜通道的化合物或其盐、溶剂化合物、立体异构体和前药。本公开还涉及合成该化合物的方法、形成合成膜通道的方法以及使用方法。具体而言,合成膜通道是位于脂质膜中的合成质子通道。
-
[EN] 2,4-DIAMINOQUINAZOLINES FOR SPINAL MUSCULAR ATROPHY<br/>[FR] 2,4-DIAMINOQUINAZOLINES UTILES POUR LE TRAITEMENT D'UNE ATROPHIE MUSCULAIRE SPINALE申请人:DECODE CHEMISTRY INC公开号:WO2005123724A1公开(公告)日:2005-12-292,4-Diaminoquinazolines of formulae I-IV and VI (I, II, III, IV and VI) are useful for treating spinal muscular atrophy (SMA).2,4-二氨基喹唑啉的化学式I-IV和VI(I,II,III,IV和VI)可用于治疗脊髓性肌萎缩症(SMA)。
-
[EN] BICYCLYL-SUBSTITUTED ISOTHIAZOLINE COMPOUNDS<br/>[FR] COMPOSÉS ISOTHIAZOLINE SUBSTITUÉS PAR UN BICYCLYLE申请人:BASF SE公开号:WO2014206910A1公开(公告)日:2014-12-31The present invention relates to bicyclyl-substituted isothiazoline compounds of formula (I) wherein the variables are as defined in the claims and description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及公式(I)中变量如索权和说明中所定义的自行车基取代异噻唑啉化合物。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种通过使用这些化合物来控制无脊椎动物害虫的方法,以及包含所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] AZOLINE COMPOUNDS<br/>[FR] COMPOSÉS AZOLINE申请人:BASF SE公开号:WO2015128358A1公开(公告)日:2015-09-03The present invention relates to azoline compounds of formula (I) wherein A, B1, B2, B3, G1, G2, X1, R1, R3a, R3b, Rg1 and Rg2 are as defined in the claims and the description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及式(I)的噁唑啉化合物,其中A、B1、B2、B3、G1、G2、X1、R1、R3a、R3b、Rg1和Rg2如权利要求和描述中所定义。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种利用这些化合物控制无脊椎动物害虫的方法,以及包括所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] SUBSTITUTED QUINAZOLINES AS FUNGICIDES<br/>[FR] QUINAZOLINES SUBSTITUÉES, UTILISÉES EN TANT QUE FONGICIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2010136475A1公开(公告)日:2010-12-02The present invention relates to a compound of formula (I) wherein wherein the substituents have the definitions as defined in claim 1or a salt or a N-oxide thereof, their use and methods for the control and/or prevention of microbial infection, particularly fungal infection, in plants and to processes for the preparation of these compounds.本发明涉及一种具有如下式(I)的化合物,其中取代基具有权利要求1中定义的定义,或其盐或N-氧化物,它们的用途以及用于控制和/或预防植物中微生物感染,特别是真菌感染的方法,以及制备这些化合物的方法。
表征谱图
-
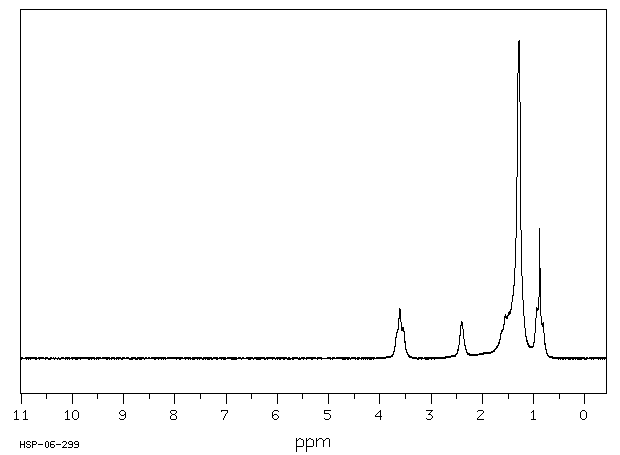
氢谱1HNMR
-
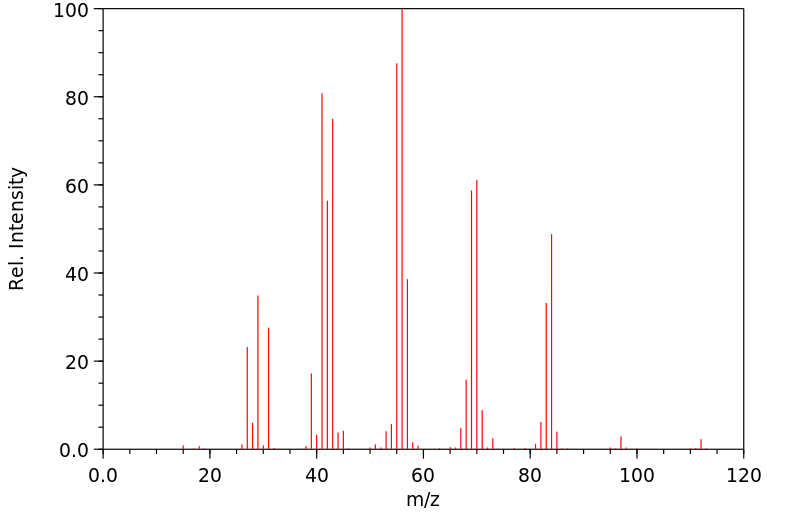
质谱MS
-
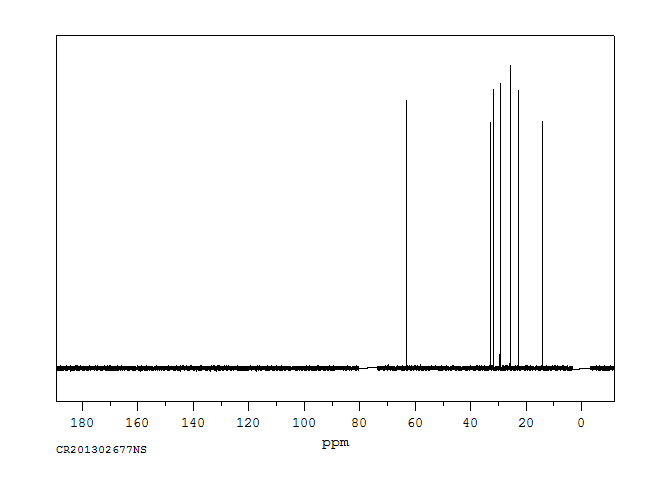
碳谱13CNMR
-
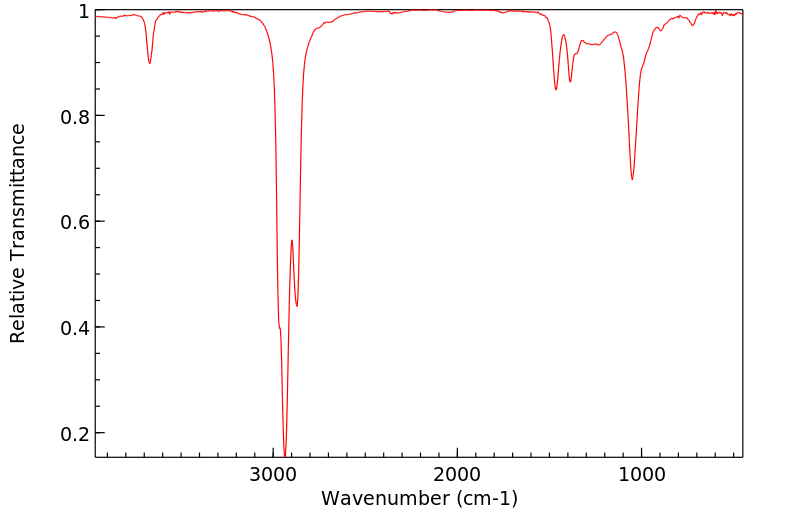
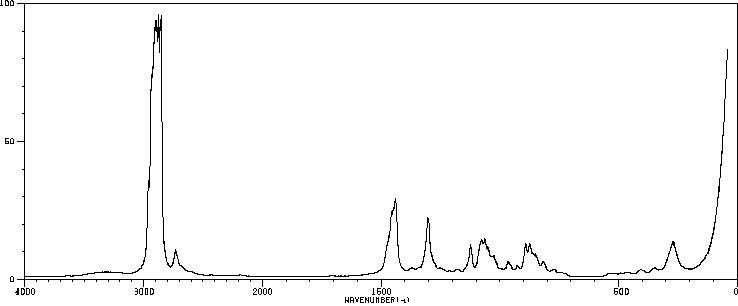
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯