莽草酸 | 138-59-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:185-187 °C (lit.)
-
比旋光度:-180 º (c=4, H2O 25 ºC)
-
沸点:225.11°C (rough estimate)
-
密度:1.52 g/cm3 (27.2℃)
-
溶解度:180克/升
-
LogP:-1.5 at 21℃ and pH1
-
表面张力:49.86mN/m at 1g/L and 20℃
-
物理描述:Solid
-
颜色/状态:Needles from methanol/ethyl acetate
-
分解:When heated to decomposition it emits acrid smoke and irritating fumes.
-
解离常数:K = 7.1X10-5 at 14.1 °C
-
碰撞截面:149.5 Ų [M+H]+ [CCS Type: DT, Method: stepped-field]
-
稳定性/保质期:
- 具有吸湿性,可以升华。
- 存在于烟叶中。
计算性质
-
辛醇/水分配系数(LogP):-1.7
-
重原子数:12
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.571
-
拓扑面积:98
-
氢给体数:4
-
氢受体数:5
ADMET
安全信息
-
危险品标志:Xi
-
安全说明:S22,S24/25
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:29181980
-
危险品运输编号:REM
-
RTECS号:GW4600000
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:应将样品充入氩气进行密封,并存放在阴凉、干燥的地方。
SDS
| Name: | Shikimic Acid 98% Material Safety Data Sheet |
| Synonym: | 3,4,5-Trihydroxy-1-Cyclohexene-1-Carboxylic Acid |
| CAS: | 138-59-0 |
Synonym:3,4,5-Trihydroxy-1-Cyclohexene-1-Carboxylic Acid
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 138-59-0 | Shikimic Acid | 98% | 205-334-2 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
Effects may be delayed. Substance may have carcinogenic potential.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam. Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 138-59-0: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 185.00 - 187.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: 18g/100ml (20 c)
Specific Gravity/Density:
Molecular Formula: C7H10O5
Molecular Weight: 174.15
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 138-59-0: GW4600000 LD50/LC50:
Not available.
Carcinogenicity:
Shikimic Acid - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 138-59-0: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 138-59-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 138-59-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
莽草酸是一种易溶于水的白色晶体粉末,因最早分离自日本莽草而得名。它作为植物和微生物的重要代谢产物,长期以来主要从八角茴香科植物(如八角或日本莽草)等干燥成熟果实中提取。近年来,也有从北美枫香果实以及松针中提取莽草酸的研究报道。
植物来源莽草酸存在于木兰科植物八角的干燥成熟果实中。八角是一种常绿乔木,高可达30米,主要分布于福建、广东、广西、云南、贵州等省的土壤疏松山地。树皮呈灰色至红褐色,椭圆形全缘单叶,披针形生长,上表面可见透明油点。春秋季开花,花单生于叶腋部,有3片黄绿色萼片和6-9片粉红至深红色花被。秋季至第二年春季结果,聚合果呈星状八角形,成熟后由绿变黄。
莽草又称野八角,为一种灌木,四季常绿,种子呈淡褐色且有光泽,主要分布在1200米以下的荒山荒坡丛林中。该植物秋季结果时果实中含有大量莽草酸,与八角类似但有毒,使用时需注意避免混淆。
性状莽草酸为白色粉末,易溶于水,在水中溶解度为18g/100ml,难溶于氯仿、苯和石油醚。熔点为185℃~191℃,旋光度-180°,气味辛酸。
生产达菲的关键原料莽草酸是罗氏生产达菲的关键原料。当前主要从八角中提取,而通过合成法或从奎宁酸(金鸡纳树的树皮)及其衍生物中制备莽草酸,由于技术复杂及成本高昂的原因,目前主要用于科研工作。
药理作用莽草酸可通过影响花生四烯酸代谢抑制血小板聚集,从而抑制动、静脉和脑血栓形成。它具有抗炎、镇痛作用,并作为抗癌药物中间体,在预防禽流感方面也有重要作用。
莽草酸途径莽草酸途径存在于植物、真菌和细菌中,通过优化酶活性位点提高代谢物流通效率。该路径的关键酶DHQ-SDH的结构研究显示,它通过凹型构造中的活性位点将代谢物区分开来,并增加其转移效率。
化学性质莽草酸易溶于水,难溶于氯仿、苯和石油醚,源自木兰科植物八角的干燥成熟果实。它是一种抗肿瘤药物,也是二恶霉素、乙二醛酶抑制剂等抗肿瘤药物的合成原料。
此外,莽草酸还是许多生物碱、芳胺酸、吲哚衍生物及手性药物合成的关键前体,并被用于奥塞米韦(达菲活性成分)的起始原料。在神经氨酸酶抑制剂领域中,可用于治疗和预防流感A和B型感染。
用途莽草酸用作定量测定根部顶端部分莽草酸含量的标准品,也可作为莽草酸激酶试验的底物。
生产方法莽草酸是从中药八角茴香中提取的一种单体化合物。
类别与安全信息莽草酸被归类为有毒物质。急性毒性测试表明,小鼠腹腔注射600毫克/公斤剂量即可致死。其可燃性较高,在火场会产生辛辣刺激烟雾。因此储存和运输时需低温、通风且干燥,并使用水、二氧化碳或干粉灭火器进行灭火。
请注意在处理该物质时应采取适当的安全防护措施,以确保人员安全及避免环境损害。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— methyl shikimate 40983-58-2 C8H12O5 188.18 —— methyl cis-3-hydroxy-4,5-epoxycyclohex-1-enecarboxylate 76985-84-7 C8H10O4 170.165 (-)-3-去氢莽草酸 (-)-3-dehydroshikimic acid 2922-42-1 C7H8O5 172.138 —— 3a,6,7,7a-tetrahydro-7-hydroxy-2,2-dimethyl-[3aR-(3aα,7α,7aα)]-1,3-benzodioxole-5-carboxylic acid 90927-40-5 C10H14O5 214.218 —— methyl 3,4-O-isopropylideneshikimate 88165-26-8 C11H16O5 228.245 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-epi-shikimic acid 171963-37-4 C7H10O5 174.153 —— methyl shikimate 40983-58-2 C8H12O5 188.18 —— (+)-methyl 3-epi-quinate —— C8H12O5 188.18 —— methyl [3S-(3α,4β,5α)]-3,4,5-trihydroxy-1-cyclohexene-1-carboxylate 171963-38-5 C8H12O5 188.18 —— methyl shikimate 14671-74-0 C8H12O5 188.18 —— 5-deoxy-shikimic acid 122554-88-5 C7H10O4 158.154 —— ethyl shikimate 101769-63-5 C9H14O5 202.207 (1S,5R,6R)-5-羟基-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸 (1S,5R,6R)-5-hydroxy-7-oxa-bicyclo[4.1.0]hept-3-ene-3-carboxylic acid 149560-23-6 C7H8O4 156.138 —— methyl cis-3-hydroxy-4,5-epoxycyclohex-1-enecarboxylate 76985-84-7 C8H10O4 170.165 —— (-)-methyl (1β,2β,6β)-2-hydroxy-7-oxabicyclo<4,1,0>hept-3-ene-4-carboxylate 106861-60-3 C8H10O4 170.165 (-)-3-去氢莽草酸 (-)-3-dehydroshikimic acid 2922-42-1 C7H8O5 172.138 —— (1S,5R,6R)-5-hydroxy-7-oxabicyclo[4.1.0]hept-3-ene-3-carboxylic acid ethyl ester —— C9H12O4 184.192 —— 5-deoxy-5-amino-shikimic acid 178948-66-8 C7H11NO4 173.169 —— (6S)-6-Fluoroshikimate 133398-72-8 C7H9FO5 192.144 莽草酸-3-磷酸酯 shikimate 3-phosphate (S3P) 63959-45-5 C7H11O8P 254.133 —— 3-amino-4,5-dihydroxy-cyclohex-1-enecarboxylic acid —— C7H11NO4 173.169 —— (-)-methyl 3-dehydroshikimate 84806-48-4 C8H10O5 186.164 —— pericosine Do 1275591-55-3 C8H11ClO5 222.625 —— 5-deoxy-S3P 135292-58-9 C7H11O7P 238.134 —— 3a,6,7,7a-tetrahydro-7-hydroxy-2,2-dimethyl-[3aR-(3aα,7α,7aα)]-1,3-benzodioxole-5-carboxylic acid 90927-40-5 C10H14O5 214.218 5-(戊烷-3-基氧基)-7-氧代-双环[4.1.0]庚-3-烯-3-羧酸乙酯 ethyl (3R,4S,5S)-4,5-epoxy-3-(1-ethylpropoxy)-cyclohex-1-ene-1-carboxylate 204254-96-6 C14H22O4 254.326 —— 3,4,5-tris-O-trimethylsilyl shikimate de methyle 91758-56-4 C17H36O5Si3 404.726 —— (3R,4S,5R)-3,4,5-triacetoxycyclohex-1-ene-1-carboxylic acid 98167-08-9 C13H16O8 300.265 —— (4S,5R)-5-hydroxy-4-methoxy-3-oxocyclohex-1-ene-1-carboxylic acid methyl ester 185810-87-1 C9H12O5 200.191 —— methyl 3,4-O-isopropylideneshikimate 88165-26-8 C11H16O5 228.245 奥司他韦杂质2 ethyl (3R,4S,5R)-5-hydroxy-3,4-isopropylidenedioxycyclohex-1-enecarboxylate 136994-78-0 C12H18O5 242.272 —— 5-O-(p-coumaroyl)shikimic acid 196496-50-1 C16H16O7 320.299 3,4-O-(二乙基甲基亚基)莽草酸乙酯 (3aR,7R,7aS)-ethyl 2,2-diethyl-7-hydroxy-3a,6,7,7a-tetrahydrobenzo[d][1,3]dioxole-5-carboxylate 943515-58-0 C14H22O5 270.326 —— methyl (3R,4S,5R)-3-hydroxy-4,5-<(2S,3S)-2,3-dimethoxybutan-2,3-dioxy>-cyclohex-1-en-1-carboxylate 245054-32-4 C14H22O7 302.324 —— methyl (3aS,4R,7aR)-4-hydroxy-3a,4,5,7a-tetrahydrospiro[benzo[d][1,3]dioxole-2,1'-cyclohexane]-6-carboxylate 120200-03-5 C14H20O5 268.31 —— (2S,3S,4aR,8S,8aR)-methyl 8-hydroxy-2,3-dimethoxy-2,3-dimethyl-2,3,4a,5,8,8a-hexahydrobenzo[b][1,4]dioxine-6-carboxylate 245054-31-3 C14H22O7 302.324 —— 4-O-caffeoylshikimic acid 1177319-06-0 C16H16O8 336.298 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:参考文献:名称:奥司他韦的疏水侧链影响神经氨酸酶抑制剂的A型亚型选择性摘要:在流感病毒生命周期中起关键作用的神经氨酸酶是新型治疗剂的靶标。结构活性关系的研究表明,奥司他韦的C-5位氨基指向第1组中的神经氨酸酶的150腔。该腔对于抑制剂针对N1和N2 NA的选择性很重要。合成了一系列流感神经氨酸酶抑制剂,在其C-5位上含有亲脂性侧链的奥司他韦支架,并评估了它们对流感神经氨酸酶的抑制活性和选择性。结果表明,化合物130(H5N1 IC 50 = 0.1±0.04μ米,H3N2 IC 50 = 0.26±0.18μ米)显示出对第1组神经氨酸酶更好的抑制活性和选择性。这项研究可能为设计更好的1组神经氨酸酶抑制剂提供线索。DOI:10.1111/cbdd.13060
-
作为产物:描述:(1S,2R,6R,7S,8R)-6-[tert-butyl(dimethyl)silyl]oxytricyclo[6.2.1.02,7]undec-9-en-3-one 在 吡啶 、 盐酸 、 甲醇 、 potassium fluoride 、 sodium hydroxide 、 sodium tetrahydroborate 、 四氧化锇 、 N-甲基吲哚酮 、 三氟甲磺酸酐 、 水 、 氧气 、 4-甲基苯磺酸吡啶 、 sodium hydride 、 1,8-二氮杂双环[5.4.0]十一碳-7-烯 、 N,N-二异丙基乙胺 、 亚磷酸三乙酯 作用下, 以 四氢呋喃 、 甲醇 、 二苯醚 、 二甲基亚砜 、 甲苯 为溶剂, 反应 307.5h, 生成 莽草酸参考文献:名称:A concise enantio- and diastereo-controlled synthesis of (−)-quinic acid and (−)-shikimic acid摘要:从(R)-4-羟基环己-2-烯酮的合成等价物开始,以对映体和非对映体控制的方式简明地合成了(â)-奎宁酸和(â)-莽草酸,它们都被认为是植物和微生物莽草酸途径中的关键中间体。DOI:10.1039/a805775c
-
作为试剂:描述:参考文献:名称:Methods for producing isomers of muconic acid and muconate salts摘要:通过提供从可再生碳源通过生物催化转化产生的顺式,反式-顺式,反式异构体的顺丁二烯二酸的方法;在反应条件下将顺式,顺式-顺丁二烯二酸异构化为顺式,反式-顺丁二烯二酸;分离顺式,反式-顺丁二烯二酸;并结晶顺式,反式-顺丁二烯二酸。顺式,反式异构体可以进一步异构为反式,反式异构体。在一个例子中,该方法包括培养表达3-脱氢植酸脱水酶,原儿茶酸脱羧酶和邻苯二酚1,2-双加氧酶的重组细胞,在包含可再生碳源的培养基中,并在可再生碳源被细胞芳香族氨基酸生物合成的共同途径中的酶将可再生碳源转化为3-脱氢植酸的条件下,将3-脱氢植酸生物催化转化为顺式,顺式-顺丁二烯二酸。公开号:US08809583B2
文献信息
-
[EN] INSULIN ANALOGUES WITH GLUCOSE REGULATED CONFORMATIONAL SWITCH<br/>[FR] ANALOGUES DE L'INSULINE À COMMUTATEUR DE CONFORMATION RÉGULÉ PAR LE GLUCOSE申请人:THERMALIN INC公开号:WO2021022116A1公开(公告)日:2021-02-04The present invention relates to glucose-responsive insulin analogues, compositions including the glucose-responsive insulin analogues, and methods of lowering blood sugar of a patient using the insulin analogue or compositions thereof.
-
CYSTOBACTAMIDES申请人:HELMHOLTZ-ZENTRUM FÜR INFEKTIONSFORSCHUNG GMBH公开号:US20160145304A1公开(公告)日:2016-05-26The present invention provides cystobactamides of formula (I) and the use thereof for the treatment or prophylaxis of bacterial infections:本发明提供了式(I)的囊胞菌素及其用于治疗或预防细菌感染的用途:
-
[EN] NEW ENDOPEROXIDE COMPOUNDS, PROCESS FOR OBTAINING THEM AND USES THEREOF FOR CONTROL OF PERKINSIOSIS IN BIVALVES<br/>[FR] NOUVEAUX COMPOSÉS ENDOPEROXYDE, LEUR PROCÉDÉ D'OBTENTION ET LEURS UTILISATIONS POUR LE CONTRÔLE DE LA PERKINSIOSE CHEZ LES BIVALVES申请人:CCMAR CENTRO DE CIENCIAS DO MAR UNIV DO ALGARVE公开号:WO2020240266A1公开(公告)日:2020-12-03The present invention relates to new endoperoxide compounds and compositions, and to a process for producing them for prophylaxis and control of perkinsiosis in bivalves. Endoperoxide compounds with biological activity against Perkinsus olseni include 13 trioxolanes and 9 tetraoxanes. Protozoan parasites of the genus Perkinsus are known to infect several species of marine molluscs worldwide, like oysters, abalones, clams, scallops, pearl oysters, cockles or mussels. The present invention also describes the synthesis of these compounds, in particular of new endoperoxide compounds of the tetraoxane family. Compositions comprising endoperoxide compounds are useful for prophylaxis and control of perkinsiosis in bivalves. Therefore, the present invention also relates to a method of controlling perkinsiosis in bivalves. The present invention is in the domain of aquaculture, medicine, pharmaceuticals and biochemistry.
-
[EN] PYRIDAZINONE COMPOUND AND HERBICIDE AND NOXIOUS ARTHROPOD CONTROLLING AGENT COMPRISING IT<br/>[FR] COMPOSÉ ET HERBICIDE À BASE DE PYRIDAZINONE ET AGENT DE LUTTE CONTRE LES ARTHROPODES NUISIBLES LE CONTENANT申请人:SUMITOMO CHEMICAL CO公开号:WO2012091156A1公开(公告)日:2012-07-05The present invention relates to a pyridazinone compound of the formula (I): wherein R1 represents hydrogen, a C1-6 alkyl group, and the like, R2 represents halogen, a cyano group, a nitro group, a C1-6 alkoxy group, and the like, G represents hydrogen, and the like, Z represents halogen, a cyano group, a nitro group, a C1-6 alkyl group, and the like, and n represents an integer of 1-5 useful as an active ingredient in a herbicideand a noxious arthropod controlling agent.
-
Novel compounds and methods for synthesis and therapy申请人:——公开号:US20040053999A1公开(公告)日:2004-03-18Novel compounds are described. The compounds generally comprise an acidic group, a basic group, a substituted amino or N-acyl and a group having an optionally hydroxylated alkane moiety. Pharmaceutical compositions comprising the inhibitors of the invention are also described. Methods of inhibiting neuraminidase in samples suspected of containing neuraminidase are also described. Antigenic materials, polymers, antibodies, conjugates of the compounds of the invention with labels, and assay methods for detecting neuraminidase activity are also described.
表征谱图
-
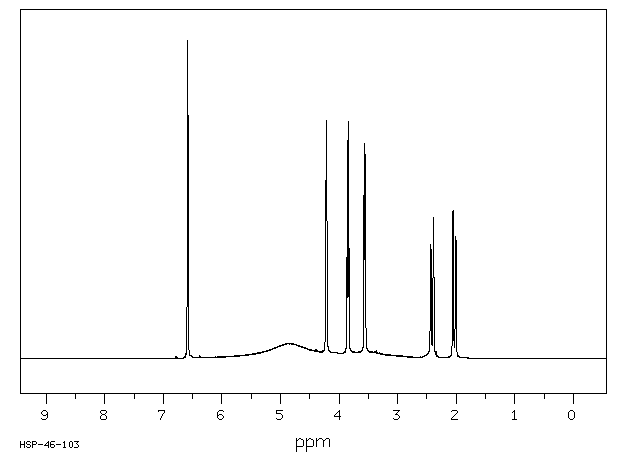
氢谱1HNMR
-
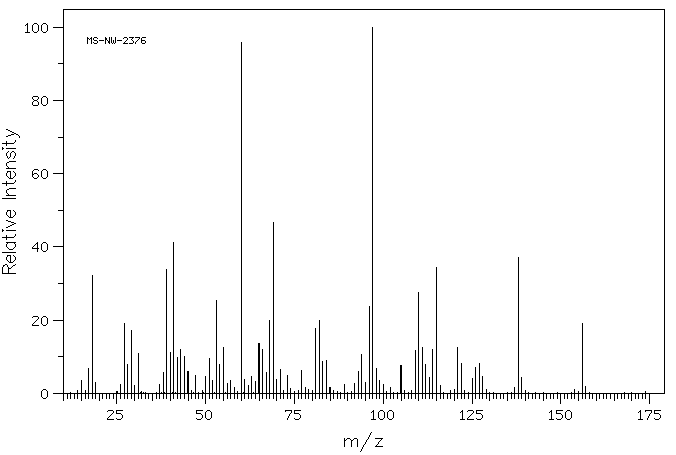
质谱MS
-
碳谱13CNMR
-
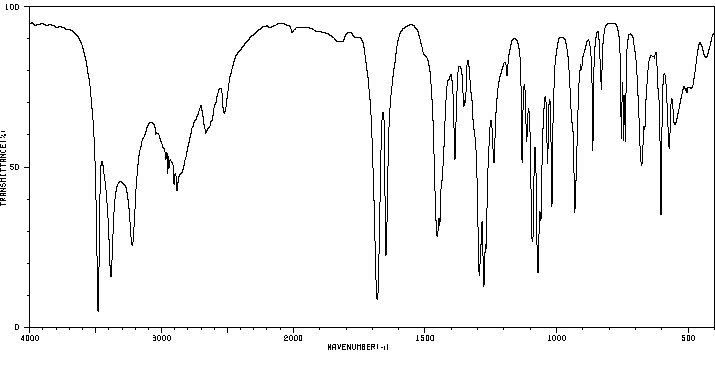
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息