苯妥英 | 57-41-0
中文名称
苯妥英
中文别名
5,5-二苯基乙内酰脲;二苯尿囊素;5,5-二苯乙内酰脲;二苯基乙內酰脲;5,5-二苯基海因;5,5-联苯基乙内酰脲
英文名称
phenythoin
英文别名
phenytoin;5,5-diphenylhydantoin;diphenylhydantoin;PHT;5,5-diphenyl-2,4-imidazolidinedione;5,5-diphenylimidazolidine-2,4-dione;dilantin;lepitoin;DPH;PHY;Sdccgsbi-0050317.P006;2-oxo-5,5-diphenyl-1H-imidazol-1-ium-4-olate
CAS
57-41-0
化学式
C15H12N2O2
mdl
——
分子量
252.272
InChiKey
CXOFVDLJLONNDW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:293-295 °C (lit.)
-
沸点:395.45°C (rough estimate)
-
密度:1.1562 (rough estimate)
-
闪点:11 °C
-
溶解度:可溶于DMSO
-
物理描述:Phenytoin appears as fine white or almost white crystalline powder. Odorless or almost odorless. Tasteless. (NTP, 1992)
-
颜色/状态:Needles (alcohol)
-
气味:Odorless
-
蒸汽压力:1.2X10-10 mm Hg at 25 °C (est)
-
稳定性/保质期:
Sensitive to light
-
分解:When heated to decomposition it emits very toxic fumes of /nitrogen oxides/.
-
Caco2细胞的药物渗透性:-4.57
-
解离常数:pKa = 8.33
-
保留指数:2310;2350;2365;2347;2308;2340;2360;2380;2306.8;2317.8;2299.2;2292;2335;2310;2385;2330
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:19
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.07
-
拓扑面积:58.2
-
氢给体数:2
-
氢受体数:2
ADMET
代谢
苯妥英广泛代谢,首先转化为反应性_亚环氧化物_中间体。据认为,这种反应性中间体是许多不希望的苯妥英不良反应的原因,如肝毒性、SJS/TEN和其他特异质反应。_亚环氧化物_代谢为_羟基苯妥英_或_苯妥英二氢二醇_代谢物,尽管前者约占苯妥英代谢的90%。有趣的是,CYP2C9和CYP2C19形成的羟基苯妥英代谢物有两种对映异构体:_(R)-p-HPPH_和_(S)-p-HPPH_。当CYP2C19催化反应时,对映异构体的比例大约为1:1,而当CYP2C9催化反应时,比例则严重偏向"S"对映异构体。由于苯妥英的代谢部分受CYP2C9和CYP2C19遗传多态性的影响,这个比例可以用来识别酶的不同基因变异。EPHX1、CYP1A2、CYP2A6、CYP2C19、CYP2C8、CYP2C9、CYP2D6、CYP2E1和CYP3A4负责产生_苯妥英二氢二醇_代谢物。_羟基苯妥英_可以通过CYP2C19、CYP3A5、CYP2C9、CYP3A4、CYP3A7、CYP2B6和CYP2D6代谢为_苯妥英儿茶酚_代谢物,或者通过UGT1A6、UGT1A9、UGT1A1和UGT1A4的葡萄糖醛酸化代谢为可以在尿液中消除的_葡萄糖醛酸代谢物_。另一方面,_苯妥英二氢二醇_实体只转化为_儿茶酚_代谢物。_儿茶酚代谢物_可以通过COMT甲基化并在尿液中随后消除,或者自发氧化为_苯妥英醌_(NQO1可以将醌转化回儿茶酚代谢物)。值得注意的是,尽管CYP2C18在肝脏中表达不良,但该酶在皮肤中活跃,并参与苯妥英的一级和二级羟基化。这种CYP2C18介导的生物激活可能与苯妥英引起的皮肤不良反应的表现有关。
Phenytoin is extensively metabolized and is first transformed into a reactive _arene oxide intermediate_. It is thought that this reactive intermediate is responsible for many undesirable phenytoin adverse effects such as hepatotoxicity, SJS/TEN, and other idiosyncratic reactions. The _arene oxide_ is metabolized to either a _hydroxyphenytoin_ or _phenytoin dihydrodiol_ metabolite, although the former accounts for about 90% of phenytoin metabolism. Interestingly, two stereoisomers of the _hydroxyphenytoin_ metabolite are formed by CYP2C9 and CYP2C19: _(R)-p-HPPH_ and _(S)-p-HPPH_. When CYP2C19 catalyzes the reaction, the ratio of stereoisomers is roughly 1:1, whereas when CYP2C9 catalyzes the reaction, the ratio heavily favours the "S" stereoisomer. Since the metabolism of phenytoin is in part influenced by genetic polymorphisms of CYP2C9 and CYP2C19, this ratio can be utilized to identify different genomic variants of the enzymes. EPHX1, CYP1A2, CYP2A6, CYP2C19, CYP2C8, CYP2C9, CYP2D6, CYP2E1 and CYP3A4 are responsible for producing the _phenytoin dihydrodiol_ metabolite. _Hydroxyphenytoin_ can be metabolized by CYP2C19, CYP3A5, CYP2C9, CYP3A4, CYP3A7, CYP2B6 and CYP2D6 to a _phenytoin catechol_ metabolite or undergo glucuronidation by UGT1A6, UGT1A9, UGT1A1, and UGT1A4 to a _glucuronide metabolite_ that can be eliminated in the urine. On the other hand, the _phenytoin dihydrodiol_ entity is only transformed to the _catechol_ metabolite. The _catechol metabolite_ can undergo methylation by COMT and be subsequently eliminated in the urine, or can spontaneously oxidize to a _phenytoin quinone_ (NQO1 can transform the quinone back to the catechol metabolite). Of note, although CYP2C18 is poorly expressed in the liver, the enzyme is active in the skin and is involved in the primary and secondary hydroxylation of phenytoin. This CYP2C18 mediated bioactivation may be linked to the manifestation of adverse cutaneous drug reactions associated with phenytoin.
来源:DrugBank
代谢
The major route of metabolism of phenytoin is oxidation by the liver to the inactive metabolite 5-(p-hydroxyphenyl)-5-phenylhydantoin (HPPH). Because this metabolism is a saturable process, small increases in dosage may produce substantial increases in plasma phenytoin concentrations...
来源:Hazardous Substances Data Bank (HSDB)
代谢
肝脏生物转化的速率在年幼儿童、孕妇、月经期女性以及急性创伤患者中会增加;随着年龄的增长,速率会降低。苯妥英的主要非活性代谢物是5-(对-羟基苯基)-5-苯基乙内酰脲(HPPH)。由于遗传倾向,苯妥英在少数个体中可能代谢缓慢,这可能导致酶活性有限和诱导不足。
The rate of hepatic biotransformation is increased in younger children, in pregnant women, in women during menses, and in patients with acute trauma; rate decreases with advancing age. The major inactive metabolite of phenytoin is 5-(p-hydroxyphenyl)-5-phenylhydantoin (HPPH). Phenytoin may be metabolized slowly in a small number of individuals due to genetic predisposition, which may cause limited enzyme availability and lack of induction.
来源:Hazardous Substances Data Bank (HSDB)
代谢
... Oxidative metabolism of 1 of geminal phenyl rings of diphenylhydantoin ... 5-meta-hydroxyphenyl-(l) and 5-para-hydroxyphenyl-5-phenylhydantoin were detected in urine of man (approx ratio 1:12) ...
来源:Hazardous Substances Data Bank (HSDB)
代谢
苯妥英已知的人类代谢物包括4-羟基苯妥英、3'-HPPH、(2S,3S,4S,5R)-6-(2,5-二氧代-4,4-二苯基咪唑烷-1-基)-3,4,5-三羟基氧杂环己烷-2-羧酸和5-(3,4-二羟基环己二烯-1-基)-5-苯基咪唑烷二酮-2,4。
Phenytoin has known human metabolites that include 4-Hydroxyphenytoin, 3'-HPPH, (2S,3S,4S,5R)-6-(2,5-dioxo-4,4-diphenylimidazolidin-1-yl)-3,4,5-trihydroxyoxane-2-carboxylic acid, and 5-(3,4-dihydroxycyclohexa-1,5-dien-1-yl)-5-phenylimidazolidine-2,4-dione.
来源:NORMAN Suspect List Exchange
毒理性
鉴定:苯妥英是一种抗癫痫药物。苯妥英是一种白色固体结晶物质或固体粉末。它无味且无臭。它可溶于水、酒精,且在氯仿、醚和二氯甲烷中几乎不溶。人体暴露:主要风险和靶器官:中毒通常表现为轻度中枢神经系统效应。在大量过量服用后可能会出现更严重的表现,但死亡极为罕见。临床效应概要:症状的出现,包括侧视性眼震颤、共济失调和嗜睡,在摄入后1至2小时内发生,可持续约4至5天。在更严重的病例中,可能会观察到水平眼震颤、粗大震颤和无法行走。在非常严重的中毒中,意识受损,但罕见昏迷。禁忌症:急性间歇性卟啉症、过敏反应。心房结性心动过缓、二度或三度房室传导阻滞、窦性心动过缓和亚当-斯托克斯综合征患者的静脉注射。在患有尿毒症、低白蛋白血症、肝功能紊乱和病毒性肝炎的患者中应谨慎使用。暴露途径:口服:最常见的途径。 parenteral:癫痫持续状态时的静脉注射。暴露途径的吸收:苯妥英从胃肠道缓慢但几乎完全吸收;吸收速率变化不定,不同药物制剂的生物利用度可能有显著差异。大剂量吸收较慢。在严重口服中毒中,胃肠道吸收可能持续长达60小时。口服给予100毫克给正常志愿者,产生了2.5至3.5小时和10至12小时的两个峰值。暴露途径的分布:苯妥英在体内广泛分布,并广泛(87至93%)与蛋白质结合。血浆结合几乎完全与白蛋白结合;在正常血浆白蛋白浓度且无置换剂的情况下,苯妥英约90%与血浆结合。暴露途径的生物半衰期:口服给药治疗剂量后,苯妥英具有非常可变的、剂量依赖性的半衰期。治疗剂量的范围是8至60小时,平均20至30小时。在成人过量服用时,范围是24至230小时。代谢:苯妥英在肝脏广泛代谢为5-(4-羟基苯基)-5-苯基-乙内酰脲,这是一种无活性的代谢物。这种对苯妥英的羟基化是由细胞色素P450 2C9进行的。这个酶还羟基化妥布特酰胺和华法林。这解释了与这些物质的相互作用。羟基化的苯妥英进一步与它的葡萄糖醛酸结合。苯妥英的羟基化是容量限制的,因为肝脏中的饱和酶系统。在治疗剂量下,代谢是非线性的(一级动力学),而在毒性剂量下,代谢是线性的(零级动力学)。羟基化的苯妥英可以被氧化为3,4-二羟基苯基-苯基乙内酰脲,这是苯妥英的儿茶酚代谢物,进一步氧化为苯妥英的3-O-甲基化儿茶酚代谢物。这些苯妥英的代谢物可能具有毒理学意义。苯妥英在儿童中代谢更快。代谢速率似乎受到遗传多态性的影响。苯妥英经历肠肝循环。暴露途径的消除:苯妥英主要通过尿液排泄其羟基化代谢物(23至70%),自由形式或结合形式(5%)。大约4%以原形从尿液中排出,5%从粪便中排出。少量从乳汁中排出。作用模式:毒动力学:苯妥英主要通过细胞色素P450系统的对羟基化消除。代谢途径在过量时受到饱和动力学的限制,允许自由苯妥英的积累。即使在治疗剂量下,以下情况下也可能积累自由苯妥英:低白蛋白血症、慢性肾衰竭、肝功能障碍、遗传性羟基化不足和其它药物抑制苯妥英的代谢。药动力学:苯妥英与电压依赖性钠通道的特定位点结合,并认为通过抑制这些电压依赖性通道的钠流,抑制神经元的持续重复放电,从而发挥其抗惊厥作用。苯妥英稳定膜,保护大脑和心脏中的钠泵。它限制了最大惊厥活动的发生,并减少了从放电焦点传播的惊厥活动,而不影响焦点本身。苯妥英具有与奎尼丁或普鲁卡因胺相似的抗心律失常特性。尽管苯妥英对心肌的电兴奋性影响最小,但它减少了收缩力,抑制了起搏器动作并改善了房室传导。它还相对于动作电位持续时间延长了有效不应期。致癌性:据报道,在母亲怀孕期间服用苯妥英的儿童中出现了神经母细胞瘤等恶性肿瘤。致畸性:苯妥英被归类为D级(正
IDENTIFICATION: Phenytoin is an antiepileptic drug. Phenytoin is a white solid crystalline substance or a solid powder. It is odorless, and tasteless. It is soluble in water, alcohol and practically insoluble in chloroform, in ether and in methylene chloride. HUMAN EXPOSURE: Main risks and target organs: The intoxication usually manifests as mild central nervous system effects. More severe manifestations may be seen following massive overdose but fatalities are extremely rare. Summary of clinical effects: Onset of symptoms including lateral nystagmus, ataxia, and drowsiness occurs within 1 to 2 hours after ingestion and may persist for about 4 to 5 days. In more severe cases, horizontal nystagmus, coarse tremor and inability to walk may be observed. In very severe poisoning, conciousness is impaired but coma is rarely observed. Contraindications: Acute intermittent porphyria, hypersensitivity. Intravenous injection in patients with sino-atrial cardiac block, second- or third degree atrio-ventricular block, sinus bradycardia and Adam-Stokes syndrome. Caution is indicated in patients with uremia, hypoalbuminemia, liver function disorders and viral hepatitis. Routes of exposure: Oral: Most common route. Parenteral: Intravenously in status epilepticus. Absorption by route of exposure: Phenytoin is slowly, but almost completely absorbed from the gastro-intestinal tract; the rate of absorption is variable and its bioavailability can differ markedly with different pharmaceutical formulations. Large doses are more slowly absorbed. In severe oral poisoning, gastro-intestinal absorption may continue up to 60 hours. Administration of 100 mg orally to normal volunteers produced two peaks at 2.5 to 3.5 and 10 to 12 hours. Distribution by route of exposure: Phenytoin is widely distributed throughout the body and is extensively (87 to 93%) bound to protein. Plasma binding is almost exclusively to albumin; in individuals with normal plasma albumin concentration and in absence of displacing agents, phenytoin is about 90% plasma bound. Biological half-life by route of exposure: Following oral administration of therapeutic doses, phenytoin has a very variable, dose-dependent half-life. The range for a therapeutic dose is from 8 to 60 hours with an average of from 20 to 30 hours. In overdose in adults the range is from 24 to 230 hours. Metabolism: Phenytoin is extensively metabolizd in the liver to 5-(4-hydroxyphenyl)-5 phenyl-hydantoin, which is inactive. This para hydroxylation of phenytoin is carried out by cytochrome P450 2C9. This enzyme also hydroxylates tolbutamide, and warfarin. This explains the interaction with these substances. The p-hydroxylated phenytoin is in turn conjugated to its glucuronide. Phenytoin hydroxylation is capacity limited because of the saturable enzyme systems in the liver. At therapeutic doses, metabolism is nonlinear (first order kinetics), while at toxic doses the metabolism is linear (zero order kinetics). The p-hydroxylated phenytoin can be oxidized to 3,4- dihydroxyphenyl-phenylhydantoin, the catechol metabolite of phenytoin, and further to the 3-O-methylated catechol metabolite of phenytoin. These metabolites of phenytoin are of possible toxicological interest. Phenytoin is more rapidly metabolised in children. The rate of metabolizm appears to be subject to genetic polymorphism. Phenytoin undergoes entero-hepatic recycling. Elimination by route of exposure: Phenytoin is mainly excreted in the urine as its hydroxylated metabolite (23 to 70%), either free or in conjugated form (5%). About 4% is excreted unchanged, in the urine and 5% in the feces. Small amounts are excreted in the milk. Mode of action: Toxicodynamics: Phenytoin is eliminated mainly through para-hydroxylation by a cytochrome P450 system. The metabolic pathway is subject to saturable kinetics in overdose, allowing accumulation of free phenytoin. Even at therapeutic doses, accumulation of free phenytoin is possible in: hypoalbuminemia, chronic renal failure, hepatic dysfunction, hereditary insufficient para-hydroxylation, and inhibition of phenytoin metabolism by other drugs. Pharmacodynamics: Phenytoin binds to specific site on voltage- dependent sodium channels and is thought to exert its anticonvulsant effect by suppressing the sustained repetitive firing of neurons by inhibiting sodium flux through these voltage dependent channels. Phenytoin stabilizes membranes, protecting the sodium pump in the brain and in the heart. It limits the development of maximal convulsive activity and reduces the spread of convulsive activity from a discharging focus without influencing the focus itself. Phenytoin has antiarrhythmic properties similar to those of quinidine or procainamide. Although phenytoin has minimal effect on the electrical excitability of cardiac muscle, it decreases the force of contraction, depresses pacemaker action and improves atrioventricular conduction. It also prolongs the effective refractory period relative to the action potential duration. Carcinogenicity: Malignancies, including neuroblastoma, in children whose mothers were on phenytoin during pregnancy have been reported. Teratogenicity: Phenytoin is classed as a teratogen risk factor D (Positive evidence of human fetal risk, but the benefits from use in pregnant women may be acceptable despite the risk). The epileptic pregnant woman taking phenytoin, either alone or in combination with other anticonvulsants, has a two to three times greater risk of delivering a child with congenital defects. It is not known if this increased risk is due to antiepileptic drugs, the disease itself, genetic factors, or a combination of these, although some evidence indicates that drugs are the causative factor. A recognizable pattern of malformations, known as the fetal hydantoin syndrome has been described and includes craniofacial and limb abnormalities, cleft lip, impaired growth, and congenital heart defects. Drugs displacing phenytoin plasma protein binding sites: Azapropazone, diazoxide, heparin, ibuprofen, phenylbutazone, salicylic acid, sulfadimethoxine, sulfafurazole, sulfamethizole, sulfamethoxydiazine, sulfamethoxypyridazine, tolbutamide and valproic acid. Decreased total and unbound plasma phenytoin concentration: caused by increased metabolism: Folic acid, Dexamethasone, Phenobarbital, Diazepam, Rifampicin, Methadone, Nitrofurantoin, Estrogens and progestagens. Increased unbound fraction of phenytoin secondary to reduced: intrinsic metabolism: Anticonvulsants: Valproic acid, Carbamazepine, Sulthiame, Clobazam, Antithrombotics: Coumarin derivatives and Triclodine, Antituberculous drugs: Isoniazid and PAS, H2 antagonists: Cimetidine, Ranitidine and Omeprazole. Non-steroidal anti-inflammatory agents: Azapropazone, Phenylbutazone, Ibuprofen.Antiinfective agents: Metronidazole and Chloramphenicol Antimycotics: Miconazole, Fluconazole. Psychoactive drugs: Fluoxetine, Risperidone . Miscellaneous: Amiodarone, Allopurinol and Disulfiram. Main adverse effects: Anticonvulsant hypersensitivity syndrome is a potentially fatal drug reaction with cutaneous and systemic manifestations. The findings are: fever, dermatological: erythema, papulous rash. In some cases erythroderma and even a lethal epidermal necrolysis has been reported. Lymphadenopathy (70%): lymphoma and depressed immunological function have been reported. Hepatitis: hepatitis may develop with severe liver failure and death. Hematological: leukocytosis with atypical lymphocytes, eosinophilia, and agranulocytosis. Connective tissues: coarsening of facial features, enlargement of the lips, gingival hyperplasia, hypertrichosis, Peyronie's disease. Clinical effects: Acute poisoning: Ingestion: Onset of symptoms and signs, principally involving the central nervous system, occurs within hours of acute overdose. These manifestations of toxicity may last many days and, in general, correlate with serum phenytoin concentrations. The earliest manifestations of toxicity following overdose are nystagmus on lateral gaze, ataxia and drowsiness. With more severe intoxication, vertical nystagmus, dysarthria, progressive ataxia to the point of inability to walk, hyperreflexia and impaired level of consciousness are observed. Coma and/or respiratory depression is rarely observed and should prompt consideration of an alternative diagnosis. Paradoxical seizures have been reported in severe phenytoin intoxication but are extremely rare. Parenteral exposure: Fatalities have been reported following intravenous administration of phenytoin to elderly patients with cardiac arrhythmias. These complications appear more likely when intravenous phenytoin is administered at a rapid rate and have been attributed to the solvent propylene glycol rather than to the phenytoin itself. The risk of hypotension and arrythmia is minimal when intravenous phenytoin is used as an anticonvulsant and administered at the recommended rate. In a series of 164 patients who received intravenous phenytoin loading following presentation with acute convulsions, the incidence of hypotension was approximately 5%, and the incidence of apnea and cardiac arrhythmias was 0%. Course, prognosis, cause of death: Normally the clinical course is one of gradual resolution of the signs and symptoms of intoxication leading to complete recovery. Death is rare after phenytoin overdose. It has been reported in association with administration of intravenous phenytoin for treatment of cardiac arrhythmias in elderly people, and rarely from coma and hypotension following oral overdose in children. Systematic description of clinical effects: Cardiovascular: Intravenous phenytoin has been reported to cause depression of cardiac conduction, ventricular fibrillation and heart block in elderly people treated for cardiac arrhythmias. Intravenous phenytoin is irritant and may cause phlebitis. Neurological: CNS: Nystagmus, ataxia, dysarthria, drowsiness, coarse resting tremor, ankle clonus, brisk deep tendon reflexes. In severe poisoning, the patient becomes obtunded, confused and disoriented. Coma and respiratory depression are unusual. Hepatic: A phenytoin hypersensitivity syndrome occurs and is characterised by hepatitis. Overall mortality rate when liver is involved is between 18% and 40%. The hepatitis is usually anicteric. Icterus indicates a poorer prognosis. Hepatomegaly with or without plenomegaly may be present. The elevated hepatic transaminases, which may be in the thousands of international units, can continue to rise after phenytoin is discontinued. Phenytoin induced chronic hepatitis has been reported. Phenytoin can induce hyperglycemia by inhibiting the release of insulin. However, hypoglycemia has been reported in a patient treated with phenytoin for 19 years who ingested 20 g phenytoin together with 225 mg zopiclone. Dermatological: In the anticonvulsant hypersensitivity syndrome, the cutaneous eruption begins as a patchy macular erythema that evolves into a dusky, pink red, confluent, papular rash that usually is pruritic. The upper trunk, face, and upper extremities are affected first, with later involvement of the lower extremities. In some cases erythroderma ensues. Patients have periorbital and facial edema. Epidermal necrolysis (even lethal) has been reported. Hematological: A number of adverse haematological effects have been reported. These are not observed following acute overdose. The hematological abnormalities reported include leucocytosis with atypical lymphocytes, eosinophilia, leucopenia and agranulocytosis. The marrow toxicity of anticonvulsants, which may be more likely when used in combination (primidone), is recognized. There may be three mechanisms of toxicity. Firstly, primidone and phenytoin both cause folate deficiency and a megaloblastic anemia. Secondly, an immune mechanism with a phenytoin-dependent antigranulocyte antibody may cause leucopenia, which resolves on discontinuing therapy. Finally, phenytoin may cause a direct toxic effect with pancytopenia and agranulocytosis. Subnormal serum folate concentrations were found in patients with chronic epilepsy treated with phenytoin. It was suggested that folate deficiency resulted from accelerated metabolism of folate consequent upon induction of liver enzymes by anticonvulsants. Immunological: It seems likely that an etiological relationship exists between phenytoin treatment and lymphoma. There is evidence of depressed immunological function in patients given phenytoin. Allergic reactions: Anticonvulsant hypersensitivity syndrome is a potentially fatal drug reaction with cutaneous and systemic manifestations to the arene oxide producing anticonvulsants: phenytoin, carbamazepine, and phenobarbital sodium. The features include fever, rash, lympheadenopathy, periorbital or facial edema, hepatitis, hematologic abnormalities, myalgia, arthralgia and pharyngitis. The reaction may be genetically determined. Other: Connective tissues: coarsening of facial features, enlargment of the lips, gingival hyperplasia and hypertrichosis. /Phenytoin/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
苯妥英钠作用于神经元细胞膜上的钠通道,限制癫痫活动的传播并减少癫痫的扩散。通过促进神经元中的钠离子外流,苯妥英钠倾向于稳定阈值,抵抗由于过度刺激或能够减少膜钠梯度的环境变化引起的过度兴奋性。这包括减少突触后的紧张性增强。失去紧张性增强可以防止皮质癫痫病灶引爆相邻的皮质区域。
Phenytoin acts on sodium channels on the neuronal cell membrane, limiting the spread of seizure activity and reducing seizure propagation. By promoting sodium efflux from neurons, phenytoin tends to stabilize the threshold against hyperexcitability caused by excessive stimulation or environmental changes capable of reducing membrane sodium gradient. This includes the reduction of post-tetanic potentiation at synapses. Loss of post-tetanic potentiation prevents cortical seizure foci from detonating adjacent cortical areas.
来源:Toxin and Toxin Target Database (T3DB)
毒理性
前瞻性研究表明,相当高比例的使用苯妥英的患者会出现暂时性的血清转氨酶升高。这些升高通常是良性的,不与肝脏组织学异常相关,即使在继续用药的情况下通常也会解决。此外,更高比例的患者会有轻到中度的γ-谷氨酰转肽酶(GGT)水平升高,这表明是肝酶诱导而不是肝脏损伤。显著转氨酶升高(>3倍升高)很少发生。
然而,重要的是,苯妥英是最常见导致临床上明显的药物诱导肝疾病和急性肝衰竭的原因之一。已发表的苯妥英(二苯乙内酰脲)引起的肝损伤案例超过100例,并描述了一种特征性的临床损伤模式(特征)。估计的发生频率从每1000人中1人到每10000人中1人,可能因种族和民族而有所不同。典型病例发生在治疗2到8周后,初期表现为发热、皮疹、面部水肿和淋巴结肿大,几天后出现黄疸和深色尿。血清酶升高可能是肝细胞型的,尽管混合模式可能更常见,罕见病例为胆汁淤积型。嗜酸性粒细胞增多、白细胞计数增加和异常淋巴细胞增多也很常见。自身抗体形成是罕见的。临床症状和体征可能模仿急性单核细胞增多症或甚至淋巴瘤(伪淋巴瘤综合征)。几乎所有苯妥英肝毒性的病例都发生在系统性超敏反应综合征的背景下,常被称为抗惊厥超敏反应综合征(HDS)或药物皮疹伴嗜酸性粒细胞增多和系统性症状综合征(DRESS)。其他表现可能是史蒂文斯-约翰逊综合征、中毒性表皮坏死松解症、再生障碍性贫血、血小板减少症、中性粒细胞减少症、肾炎和肺炎。大多数肝损伤的病例是自限性的,在停止苯妥英后1到2个月内解决。然而,肝脏损伤可能是严重的,已经报道了许多致命的实例,苯妥英通常出现在药物诱导急性肝衰竭的前10位原因中。在典型病例中,恢复通常是完全的。
可能性评分:A(临床上明显肝损伤的已知原因)。
Prospective studies indicate that a fairly high proportion of patients taking phenytoin have transient serum aminotransferase elevations. These elevations are usually benign, not associated with liver histological abnormalities and usually resolve even with drug continuation. In addition, a higher proportion of patients have mild-to-moderate elevations in gammaglutamyl transpeptidase (GGT) levels, which is indicative of hepatic enzyme induction rather than liver injury. Marked aminotransferase elevations (>3 fold elevated) occur rarely.
Importantly, however, phenytoin is one of the most common causes of clinically apparent drug induced liver disease and acute liver failure. More than 100 cases of liver injury due to phenytoin (diphenylhydantoin) have been published and a characteristic clinical pattern (signature) of injury has been described. The estimated frequency ranges from 1 per 1000 to 1 per 10,000 and probably varies by race and ethnicity. The typical case arises after 2 to 8 weeks of therapy with initial onset of fever, rash, facial edema and lymphadenopathy, followed in a few days by jaundice and dark urine. The serum enzyme elevations can be hepatocellular, although mixed patterns are probably more common and rare cases are cholestatic. Eosinophilia, increased white counts and atypical lymphocytosis are also common. Autoantibody formation is rare. The clinical symptoms and signs can mimic acute mononucleosis or even lymphoma (pseudo-lymphoma syndrome). Almost all cases of phenytoin hepatotoxicity occur in the context of a systemic hypersensitivity syndrome and it is referred to often as the anticonvulsant hypersensitivity syndrome (HDS) or drug rash with eosinophilia and systemic symptoms syndrome (DRESS). Other manifestations can be Stevens-Johnson syndrome, toxic epidermal necrolysis, aplastic anemia, thrombocytopenia, neutropenia, nephritis, and pneumonitis. Most cases of liver injury are self-limiting and resolve within 1 to 2 months of stopping phenytoin. However, the liver injury can be severe and many fatal instances have been reported, phenytoin usually appearing in the top 10 causes of drug induced acute liver failure. In the typical case, however, recovery is usually complete.
Likelihood score: A (well known cause of clinically apparent liver injury).
来源:LiverTox
毒理性
药物性肝损伤标注:最令人关注的药物性肝损伤
DILI Annotation:Most-DILI-Concern
来源:Drug Induced Liver Injury Rank (DILIrank) Dataset
吸收、分配和排泄
鉴于其狭窄的治疗指数,建议进行治疗药物监测以帮助指导用药剂量。苯妥英钠可被完全吸收。快速释放制剂和缓释制剂给药后,分别在约1.5-3小时和4-12小时达到血浆峰浓度。需要注意的是,在急性摄入情况下,吸收可能会明显延长。
Given its narrow therapeutic index, therapeutic drug monitoring is recommended to help guide dosing. Phenytoin is completely absorbed. Peak plasma concentration is attained approximately 1.5-3 hours, and 4-12 hours after administration of the immediate release formulation and the extended release formulation, respectively. It should be noted that absorption can be markedly prolonged in situations of acute ingestion.
来源:DrugBank
吸收、分配和排泄
The majority of phenytoin is excreted as inactive metabolites in the bile. An estimated 1-5% of phenytoin is eliminated unchanged in the urine.
来源:DrugBank
吸收、分配和排泄
据报道,苯妥英的分布容积大约为0.75升/千克。
The volume of distribution of phenytoin is reported to be approximately 0.75 L/kg.
来源:DrugBank
吸收、分配和排泄
苯妥英的清除是非线性的。在较低的血药浓度下(小于10 mg/L),消除表现为一级动力学特征。随着血药浓度的增加,动力学逐渐向零级转变,一旦系统饱和,最终达到零级动力学。
The clearance of phenytoin is non-linear. At lower serum concentrations (less than 10 mg/L), elimination is characterized by first order kinetics. As plasma concentrations increase, the kinetics shift gradually towards zero-order, and finally reach zero-order kinetics once the system is saturated.
来源:DrugBank
吸收、分配和排泄
Studies using Dilantin have shown that phenytoin and its sodium salt are usually completely absorbed from the GI tract. Bioavailability may vary enough among oral phenytoin sodium preparations of different manufacturers to result in toxic serum concentrations or a loss of seizure control (subtherapeutic serum concentrations)...
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
危险等级:6.1(b)
-
危险品标志:Xn,F,T
-
安全说明:S16,S36/37,S45,S53,S7
-
危险类别码:R20/21/22,R63,R61,R40,R22,R45,R11,R23/24/25,R39/23/24/25
-
WGK Germany:3
-
海关编码:29332100
-
危险品运输编号:2811
-
RTECS号:MU1050000
-
包装等级:II
-
危险类别:6.1(b)
-
危险标志:GHS07,GHS08
-
危险性描述:H302,H350,H360
-
危险性防范说明:P201,P280,P301 + P312 + P330,P308 + P313
-
储存条件:本品应密封避光保存。
SDS
5,5-二苯基乙内酰脲 修改号码:5
模块 1. 化学品
产品名称: 5,5-DiphenylhydaNToin
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第4级
生殖细胞敏感性 1B类
致癌性 第2级
生殖毒性 1A类
特异性靶器官毒性 神经系统
- 单一接触 [第1级]
特异性靶器官毒性 肝脏, 神经系统, 牙龈, 淋巴腺
- 单一接触 [第1级]
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 吞咽有害。
可能导致遗传性缺陷
怀疑会致癌
可能损害生育能力或胎儿
对器官引起损害: 神经系统
可能因延长或接触对器官产生损害: 肝脏 神经系统 牙龈
淋巴腺
防范说明
5,5-二苯基乙内酰脲 修改号码:5
模块 2. 危险性概述
[预防] 使用前获取特定手册。
处理前必须阅读并理解所有安全措施。
切勿吸入。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
使用个人所需的防护用具。
[急救措施] 食入:若感不适,呼叫解毒中心/医生。漱口。
如接触到或相关接触:求医/就诊。
[储存] 存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 5,5-二苯基乙内酰脲
百分比: >99.0%(T)
CAS编码: 57-41-0
俗名: Phenytoin
分子式: C15H12N2O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。
求医/就诊。
食入: 求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(针对有毒颗粒的P3过滤式空气呼吸器)。远离溢出物/泄露
紧急措施: 处并处在上风处。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果粉尘或浮质产生,使用局部排气。
5,5-二苯基乙内酰脲 修改号码:5
模块 7. 操作处置与储存
操作处置注意事项: 避免所有部位的接触!
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点: 298°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 不溶于
[其他溶剂]
微溶于: 酒精, 丙酮
不溶于: 醚, 苯, 氯仿
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: ivn-rat LD50:101 mg/kg
orl-rat LD50:1635 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: dna-esc 50 umol/L
dni-hmn-lym 360 umol/L
致癌性:
5,5-二苯基乙内酰脲 修改号码:5
模块 11. 毒理学信息
IARC = 2B (怀疑对人类致癌)。
NTP = b(合理预期致癌物)
生殖毒性: 无资料
RTECS 号码: MU1050000
模块 12. 生态学信息
生态毒性:
鱼类: 96h LC50:>16.0 mg/L (Oryzias latipes)
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 1 % (by BOD), 2 % (by HPLC)
潜在生物累积 (BCF): 1.7 (conc. 0.1 mg/L), <5.0 (conc. 0.01 mg/L)
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 5,5-DiphenylhydaNToin
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第4级
生殖细胞敏感性 1B类
致癌性 第2级
生殖毒性 1A类
特异性靶器官毒性 神经系统
- 单一接触 [第1级]
特异性靶器官毒性 肝脏, 神经系统, 牙龈, 淋巴腺
- 单一接触 [第1级]
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 吞咽有害。
可能导致遗传性缺陷
怀疑会致癌
可能损害生育能力或胎儿
对器官引起损害: 神经系统
可能因延长或接触对器官产生损害: 肝脏 神经系统 牙龈
淋巴腺
防范说明
5,5-二苯基乙内酰脲 修改号码:5
模块 2. 危险性概述
[预防] 使用前获取特定手册。
处理前必须阅读并理解所有安全措施。
切勿吸入。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
使用个人所需的防护用具。
[急救措施] 食入:若感不适,呼叫解毒中心/医生。漱口。
如接触到或相关接触:求医/就诊。
[储存] 存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 5,5-二苯基乙内酰脲
百分比: >99.0%(T)
CAS编码: 57-41-0
俗名: Phenytoin
分子式: C15H12N2O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。
求医/就诊。
食入: 求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(针对有毒颗粒的P3过滤式空气呼吸器)。远离溢出物/泄露
紧急措施: 处并处在上风处。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果粉尘或浮质产生,使用局部排气。
5,5-二苯基乙内酰脲 修改号码:5
模块 7. 操作处置与储存
操作处置注意事项: 避免所有部位的接触!
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点: 298°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 不溶于
[其他溶剂]
微溶于: 酒精, 丙酮
不溶于: 醚, 苯, 氯仿
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: ivn-rat LD50:101 mg/kg
orl-rat LD50:1635 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: dna-esc 50 umol/L
dni-hmn-lym 360 umol/L
致癌性:
5,5-二苯基乙内酰脲 修改号码:5
模块 11. 毒理学信息
IARC = 2B (怀疑对人类致癌)。
NTP = b(合理预期致癌物)
生殖毒性: 无资料
RTECS 号码: MU1050000
模块 12. 生态学信息
生态毒性:
鱼类: 96h LC50:>16.0 mg/L (Oryzias latipes)
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 1 % (by BOD), 2 % (by HPLC)
潜在生物累积 (BCF): 1.7 (conc. 0.1 mg/L), <5.0 (conc. 0.01 mg/L)
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
苯妥英
苯妥英是一种抗癫痫药物,用于治疗多种类型的癫痫发作。它也归类为Vaughan-WilliamsⅠB类抗心律失常药物,但目前在临床上较少用于心脏节律异常的治疗。
适应症苯妥英主要适用于强直阵挛性发作和部分性发作的治疗,但对失神性发作无效。该药可以通过口服或静脉注射给药,尤其是在癫痫持续状态时,静脉注射苯妥英比苯二氮卓类药物更为有效。
副作用常见的副作用包括恶心、胃痛、食欲减退、肢体协调障碍、毛发增多以及牙龈增生。其他可能的严重副作用还包括嗜睡、自残行为、肝脏问题、骨髓抑制、低血压及中毒性表皮坏死松解症。
生物活性Phenytoin(二苯甲酰胺)是一种失活的电压门控钠离子通道稳定剂。
靶点- Sodium channel
Phenytoin 是一种抗惊厥药物,主要用于治疗部分发作和全面性强直阵挛性发作,而不适用于原发性全身发作如失神发作或肌阵挛发作。苯妥英通过抑制电压门控钠通道发挥作用,它在去极化膜电位时对静息态钠通道亲和力较低,在去极化并使通道进入开放和失活状态时则表现出更强的结合和阻断作用。这种阻断具有高度使用依赖性,因此在长时间或重复激活期间积累,如癫痫发作时。苯妥英对快速钠电流的作用缓慢,不改变药物存在的条件下的快钠电流时间进程,并且能选择性地抑制癫痫中的病理性超兴奋而不严重影响正在进行的活动。此外,苯妥英还阻断持续钠电流,这对控制癫痫尤其重要。Phenytoin 是一类 1b 抗心律失常药。
体内研究体外研究表明,苯妥英(5,5-二苯甲酰胺;60 mg/kg;每日给药28天)在六周龄的雌性Rag2-/- Il2rg-/-小鼠中可减少MDA-MB-231细胞引起的肿瘤生长。
用途用作抗癫痫药物和抗心律失常药物。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-(羟基甲基)苯妥英 3-hydroxymethyl-5,5-diphenyl-imidazolidine-2,4-dione 21616-46-6 C16H14N2O3 282.299 —— 5,5-diphenyl-N-acetylhydantoin 1037-93-0 C17H14N2O3 294.31 5,5-二苯基-2-硫代海因 5,5-diphenyl-2-thioxoimidazolidin-4-one 21083-47-6 C15H12N2OS 268.339 —— (2,5-dioxo-4,4-diphenylimidazolidin-1-yl)methyl acetate 27506-79-2 C18H16N2O4 324.336 N,N-二氯苯妥英 1,3-dichloro-5,5-diphenylhydantoin 100965-46-6 C15H10Cl2N2O2 321.163 N-乙氧羰基苯妥英 ethyl 3-(5,5-diphenylhydantoin) formate 1097-57-0 C18H16N2O4 324.336 —— 1-(Acetamidoacetyl)-5,5-diphenylimidazolidine-2,4-dione 170700-20-6 C19H17N3O4 351.362 —— 1-(N,N-Bis-acetylaminoacetyl)-5,5-diphenylimidazolidine-2,4-dione 170700-18-2 C21H19N3O5 393.399 2,2-二苯基海因酸 2.2-Diphenylhydantoinsaeure 6802-95-5 C15H14N2O3 270.288 苯妥英钠杂质E α-amino-α,α-diphenylacetamide 15427-81-3 C14H14N2O 226.278 5,5-二(苯基)咪唑烷-2,4-二硫酮 Thiophenytoin 2032-13-5 C15H12N2S2 284.406 —— N-<(5,5-Diphenyl)-4-oxo-2-imidazolidinyl>glycine 170700-19-3 C17H15N3O3 309.324 —— 5,5-Diphenyl-2,3,5,6-tetrahydroimidazo<2,1-b>imidazoline-3,6-dione 154748-98-8 C17H13N3O2 291.309 —— 2-methyl-5,5-diphenyl-3,5-dihydro-imidazol-4-one 24133-90-2 C16H14N2O 250.3 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5-(4-羟基苯基)-5-苯基海因 5-(4-hydroxyphenyl)-5-phenylhydantoin 2784-27-2 C15H12N2O3 268.272 3-甲基-5,5-二苯基海因 3-methyl-5,5-diphenyl-imidazolidine-2,4-dione 4224-00-4 C16H14N2O2 266.299 —— 5-phenyl-5-(4-(trifluoromethoxy)phenyl)imidazolidine-2,4-dione —— C16H11F3N2O3 336.27 4-(3-羟基苯基)-4-苯基全氢咪唑-2,5-二酮 3'-Hydroxyphenytoin 30074-03-4 C15H12N2O3 268.272 苯妥英钠杂质8 5-(4-nitrophenyl)-5-phenylimidazolidine-2,4-dione 56079-91-5 C15H11N3O4 297.27 1-甲基-5,5-二苯基-2,4-咪唑烷二酮 1-methyl-5,5-diphenylimidazolidine-2,4-dione 6859-11-6 C16H14N2O2 266.299 3-氨基-5,5-二(苯基)咪唑烷-2,4-二酮 3-amino-5,5-diphenylimidazolidine-2,4-dione 1224-08-4 C15H13N3O2 267.287 —— 3-ethyl-5,5-diphenylhydantoin 39588-47-1 C17H16N2O2 280.326 —— 3-(chloromethyl)-5,5-diphenylhydantoin 93360-07-7 C16H13ClN2O2 300.744 —— 3-((dimethylamino)methyl)-5,5-diphenylimidazolidine-2,4-dione 6507-58-0 C18H19N3O2 309.368 3-(羟基甲基)苯妥英 3-hydroxymethyl-5,5-diphenyl-imidazolidine-2,4-dione 21616-46-6 C16H14N2O3 282.299 4,4-二苯基-咪唑烷-2-酮 4,4-diphenyl-imidazolidin-2-one 103272-79-3 C15H14N2O 238.289 3-(2-氨基乙基)-5,5-二苯基乙内酰脲 3-(2-aminoethyl)-5,5-diphenylhydantoin 14046-88-9 C17H17N3O2 295.341 —— 3-((diethylamino)methyl)-5,5-diphenylimidazolidine-2,4-dione 854-77-3 C20H23N3O2 337.422 —— 5,5-diphenyl-N-acetylhydantoin 1037-93-0 C17H14N2O3 294.31 5,5-二苯基-3-丙基咪唑烷-2,4-二酮 5,5-diphenyl-3-propyl-imidazolidine-2,4-dione 62053-81-0 C18H18N2O2 294.353 —— 5,5-diphenyl-3-(prop-2-yn-1-yl)imidazolidine-2,4-dione 2718-07-2 C18H14N2O2 290.321 —— 2-(2,5-Dioxo-4,4-diphenylimidazolidin-1-yl)acetonitrile —— C17H13N3O2 291.309 —— 3-<2-Hydroxy-aethyl>-5.5-diphenyl-hydantoin 43016-30-4 C17H16N2O3 296.326 —— 3-benzyl-5,5-diphenyl-imidazolidine-2,4-dione 34657-67-5 C22H18N2O2 342.397 —— 3-(2-chloroethyl)-5,5-diphenyl-imidazolidine-2,4-dione 54742-84-6 C17H15ClN2O2 314.771 —— 3-allyl-5,5-diphenylimidazolidine-2,4-dione —— C18H16N2O2 292.337 3-[(4-甲基哌嗪-1-基)甲基]-5,5-二(苯基)咪唑烷-2,4-二酮 3-((4-methylpiperazin-1-yl)methyl)-5,5-diphenylimidazolidine-2,4-dione 21598-58-3 C21H24N4O2 364.447 1,3-二甲基-5,5-二苯基-2,4-咪唑烷二酮 1,3-dimethyl-5,5-diphenylhydantoin 6456-01-5 C17H16N2O2 280.326 —— 3-(2-dimethylamino-ethyl)-5,5-diphenyl-imidazolidine-2,4-dione 54742-79-9 C19H21N3O2 323.395 3-(2-溴乙基)-5,5-二苯基咪唑烷-2,4-二酮 3-(2-bromoethyl)-5,5-diphenylhydantoin 13272-33-8 C17H15BrN2O2 359.222 —— 3-[(4-Methylphenyl)methyl]-5,5-diphenylimidazolidine-2,4-dione 850029-94-6 C23H20N2O2 356.424 —— 3-<2-Diaethylamino-aethyl>-5.5-diphenyl-hydantoin 34341-24-7 C21H25N3O2 351.448 —— 3-butyl-5,5-diphenyl-imidazolidine-2,4-dione 62024-29-7 C19H20N2O2 308.38 —— N(3),N'(3)-ethylene-bis-5,5-diphenylhydantoin 13272-37-2 C32H26N4O4 530.583 —— 3-isobutyl-5,5-diphenylhydantoin 924047-00-7 C19H20N2O2 308.38 —— 5,5-diphenyl-3-methylthiomethylhydantoin 66503-15-9 C17H16N2O2S 312.392 5-(2-羟基苯基)-5-苯基咪唑烷-2,4-二酮 2'-Hydroxyphenytoin 60919-11-1 C15H12N2O3 268.272 —— 3-(3-dimethylamino-propyl)-5,5-diphenyl-imidazolidine-2,4-dione 109399-11-3 C20H23N3O2 337.422 —— 3-Pentyl-5,5-diphenylimidazolidine-2,4-dione 102181-51-1 C20H22N2O2 322.407 —— 3-[(4-Chlorophenyl)methyl]-5,5-diphenylimidazolidine-2,4-dione 749887-67-0 C22H17ClN2O2 376.842 —— 3-tert-butyl-5,5-diphenyl-2,4-imidazolidinedione 93504-25-7 C19H20N2O2 308.38 —— 2-(2,5-dioxo-4,4-diphenyl-imidazolidin-1-yl)-acetamide 741-27-5 C17H15N3O3 309.324 —— 3,3'-(1,6-hexanediyl)bis(5,5-diphenyl-2,4-imidazolinedione) 105859-87-8 C36H34N4O4 586.69 —— 4-[(2,5-dioxo-4,4-diphenyl-1-imidazolidinyl)methyl]benzonitrile 743468-93-1 C23H17N3O2 367.407 —— 3-decyl-5,5-diphenylimidazolidine-2,4-dione —— C25H32N2O2 392.541 —— 3-(morpholinomethyl)-5,5-diphenylimidazolidine-2,4-dione 856-85-9 C20H21N3O3 351.405 —— 3-octyl-5,5-diphenylimidazolidine-2,4-dione —— C23H28N2O2 364.488 —— 3-[(4-Bromophenyl)methyl]-5,5-diphenylimidazolidine-2,4-dione 727701-30-6 C22H17BrN2O2 421.293 —— 3-(4-t-Butylbenzyl)-5,5-diphenyl-2,4-imidazolidinedione 727714-10-5 C26H26N2O2 398.505 —— 3-(3-diisopropylamino-propyl)-5,5-diphenyl-imidazolidine-2,4-dione 114279-62-8 C24H31N3O2 393.529 5,5-二苯基-3-(3-哌啶-1-基丙基)咪唑烷-2,4-二酮 5,5-diphenyl-3-(3-piperidin-1-yl-propyl)-imidazolidine-2,4-dione 53967-10-5 C23H27N3O2 377.486 —— 3-N-(2-morpholinoethyl)-5,5-diphenylhydantoin 20000-16-2 C21H23N3O3 365.432 —— 5,5-diphenyl-3-(2-piperidin-1-yl-ethyl)-imidazolidine-2,4-dione 63381-77-1 C22H25N3O2 363.459 —— 5,5-diphenyl-3-(1,2,5,6-tetrahydropyridin-1-ylmethyl)imidazole-2,4-dione 86254-07-1 C21H21N3O2 347.417 —— 9-(2,5-Dioxo-4,4-diphenylimidazolidin-1-yl)nonanoic acid 143547-57-3 C24H28N2O4 408.497 —— N-benzyl-2-(2,5-dioxo-4,4-diphenylimidazolidin-1-yl)acetamide —— C24H21N3O3 399.449 —— N-benzyl-3-(2,5-dioxo-4,4-diphenylimidazolidin-1-yl)propanamide —— C25H23N3O3 413.476 —— (2,5-dioxo-4,4-diphenylimidazolidin-1-yl)methyl acetate 27506-79-2 C18H16N2O4 324.336 —— 3-(2-oxo-2-phenylethyl)-5,5-diphenylimidazolidine-2,4-dione —— C23H18N2O3 370.408 —— 5,5-diphenyl-3-(p-tolylaminoethyl)imidazolidine-2,4-dione 1346457-58-6 C24H23N3O2 385.466 —— 1-Acetyl-5,5-diphenylhydantoin 4635-21-6 C17H14N2O3 294.31 —— 2,4-Imidazolidinedione, 3-((1-oxopropoxy)methyl)-5,5-diphenyl- 85901-32-2 C19H18N2O4 338.363 —— 2-(2,5-dioxo-4,4-diphenylimidazolidin-1-yl)-N-(4-fluorobenzyl)acetamide 746645-05-6 C24H20FN3O3 417.44 —— ethyl 2-(2,5-dioxo-4,4-diphenylimidazolidin-1-yl)acetate 976-85-2 C19H18N2O4 338.363 磷苯妥英 fosphenytoin 93390-81-9 C16H15N2O6P 362.279 —— 3-(2,5-dioxo-4,4-diphenylimidazolidin-1-yl)-N-(4-fluorobenzyl)propanamide —— C25H22FN3O3 431.466 —— 3-(2-(N-benzyl-N-methyl-amino)-2-oxo-ethyl)-5,5-diphenylimidazolidine-2,4-dione —— C25H23N3O3 413.476 —— 3-(hydroxymethyl)-5,5-diphenylhydantoin N,N-dimethylglycine ester 71919-14-7 C20H21N3O4 367.404 —— 3-[2-(4-fluorophenylamino)ethyl]-5,5-diphenylimidazolidine-2,4-dione 1346457-56-4 C23H20FN3O2 389.429 —— 3-[2-(4-chlorophenylamino)ethyl]-5,5-diphenylimidazolidine-2,4-dione 1346457-55-3 C23H20ClN3O2 405.884 —— 3-[2-(4-hydroxyphenylamino)ethyl]-5,5-diphenylimidazolidine-2,4-dione 1346457-54-2 C23H21N3O3 387.438 —— 3-(β-diethylamino-isopropyl)-5,5-diphenyl-imidazolidine-2,4-dione 102477-28-1 C22H27N3O2 365.475 5,5-二苯基-3-[3-(4-苯基哌啶-1-基)丙基]咪唑烷-2,4-二酮 MA 1598 56079-61-9 C29H31N3O2 453.584 —— 3-(4-nitro-benzyl)-5,5-diphenyl-imidazolidine-2,4-dione 51323-55-8 C22H17N3O4 387.395 —— Phenytoin,3-CH2OC(=O)CH2CH2CH3 85901-33-3 C20H20N2O4 352.39 —— 5,5-diphenyl-3-((thiazol-2-yl-amino)methyl)imidazolidine-2,4-dione 1308827-46-4 C19H16N4O2S 364.428 —— 5-phenyl-5-phenyl-3-(oxiran-2-ylmethyl)imidazolidine-2,4-dione —— C18H16N2O3 308.337 5,5-二苯基-3-(三氯甲基硫代)海因 5,5-diphenyl-3-trichloromethylsulfanyl-imidazolidine-2,4-dione 53743-19-4 C16H11Cl3N2O2S 401.701 3-(3,4-二氢异喹啉-2(1H)-基甲基)-5,5-diphenylimidazolidine-2,4-二酮 5,5-diphenyl-3-(1,2,3,4-tetrahydroisoquinolin-2-ylmethyl)imidazole-2,4-dione 86254-08-2 C25H23N3O2 397.477 —— 3-pentanoyloxymethyl 5,5-diphenylhydantoin 85901-34-4 C21H22N2O4 366.417 —— 2-(2,5-dioxo-4,4-diphenylimidazolidin-1-yl)-N-(4-methoxybenzyl)acetamide 749919-66-2 C25H23N3O4 429.475 —— 5,5-diphenyl-3-methylsulfonylmethylhydantoin 113296-59-6 C17H16N2O4S 344.391 —— 3-(2-(4-fluorophenyl)-2-oxoethyl)-5,5-diphenylimidazolidine-2,4-dione —— C23H17FN2O3 388.398 —— 3-cyclohexyl-5,5'-diphenyl-imidazolidine-2,4-dione 535993-50-1 C21H22N2O2 334.418 —— 5,5-Diphenyl-3-[(pyridin-2-ylamino)methyl]imidazolidine-2,4-dione 87447-35-6 C21H18N4O2 358.4 —— 3-(2-(4-chlorophenyl)-2-oxoethyl)-5,5-diphenylimidazolidine-2,4-dione —— C23H17ClN2O3 404.853 —— 3-[2-(4-methoxyphenylamino)ethyl]-5,5-diphenylimidazolidine-2,4-dione 1346457-57-5 C24H23N3O3 401.465 —— 3-(2,5-dioxo-4,4-diphenylimidazolidin-1-yl)-N-(4-methoxybenzyl)propanamide —— C26H25N3O4 443.502 —— 5,5-diphenyl-3-((4-phenylpiperazin-1-yl)methyl)imidazolidine-2,4-dione 21598-65-2 C26H26N4O2 426.518 N,N-二氯苯妥英 1,3-dichloro-5,5-diphenylhydantoin 100965-46-6 C15H10Cl2N2O2 321.163 —— (2,5-Dioxo-4,4-diphenylimidazolidin-1-yl)methyl heptanoate 85901-37-7 C23H26N2O4 394.47 —— 3-octanoyloxymethyl 5,5-diphenylhydantoin 85901-38-8 C24H28N2O4 408.497 1,3-二溴-5,5-二苯基咪唑烷-2,4-二酮 1,3-dibromo-5,5-diphenylimidazolidine-2,4-dione 92426-30-7 C15H10Br2N2O2 410.065 —— 2-(2,5-dioxo-4,4-diphenylimidazolidin-1-yl)-N-phenylacetamide 748-96-9 C23H19N3O3 385.422 —— 3-(2,5-dioxo-4,4-diphenylimidazolidin-1-yl)-N-phenylpropanamide —— C24H21N3O3 399.449 —— 3-(2-(4-methoxyphenyl)-2-oxoethyl)-5,5-diphenylimidazolidine-2,4-dione —— C24H20N2O4 400.434 —— 3-(1-benzyl-piperidin-4-yl)-5,5-diphenyl-imidazolidine-2,4-dione 895148-72-8 C27H27N3O2 425.53 —— 5,5-diphenyl-3-[2-(4-p-tolylpiperazin-1-yl)ethyl]imidazolidine-2,4-dione 78807-25-7 C28H30N4O2 454.572 —— 3-((1H-imidazol-1-yl)methyl)-5,5-diphenylimidazolidine-2,4-dione 1308827-43-1 C19H16N4O2 332.362 —— N-[2-(diethylamino)ethyl]-4-[(2,5-dioxo-4,4-diphenylimidazolidin-1-yl)methylamino]benzamide 87447-30-1 C29H33N5O3 499.6 —— 3-{2-[4-(4-fluorophenyl)piperazin-1-yl]ethyl}-5,5-diphenylimidazolidine-2,4-dione 1346457-50-8 C27H27FN4O2 458.535 —— 3-(2,5-dioxo-4,4-diphenylimidazolidin-1-yl)-N-(4-fluorophenyl)propanamide —— C24H20FN3O3 417.44 —— 3-{2-[4-(4-hydroxyphenyl)piperazin-1-yl]ethyl}-5,5-diphenylimidazolidine-2,4-dione 1346457-49-5 C27H28N4O3 456.544 —— 2-(2,5-dioxo-4,4-diphenylimidazolidin-1-yl)-N-(4-sulfamoylbenzyl)acetamide —— C24H22N4O5S 478.528 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
反应信息
-
作为反应物:参考文献:名称:苯妥英前药III:口服和/或肠胃外使用的水溶性前药。摘要:合成了苯妥英钠的各种生物可逆衍生物,苯妥英钠是口服和胃肠外给药后水溶性差且易失性的药物。对这些预期的前药的初步评估,即它们的水溶性,在各种动物组织中的裂解以及在小鼠中的抗惊厥活性,证实了许多衍生物确实起了前药的作用。最有前途的前药是磷酸二钠酯和3-(羟甲基)-5,5-二苯基乙内酰脲的各种含氨基的酰基酯。DOI:10.1002/jps.2600730812
-
作为产物:参考文献:名称:Palladium Catalyzed C-Arylation of Amino Acid Derived Hydantoins摘要:Palladium(II) trifluoroacetate (5 mol %) catalyzes the C-arylation of N,N-disubstituted hydantoins by aryl iodides in good yield. The reaction proceeds through base-promoted enolization of the amino acid derived hydantoins, and the resulting 5,5-disubstituted hydantoins may be deprotected at one or both N atoms to yield biologically active structures or alternatively hydrolyzed to the parent a-aryl a-amino acids. The reaction is successful with a variety of parent amino acids and a range of electron-rich and electron-poor aryl iodides.DOI:10.1021/acs.orglett.5b01803
-
作为试剂:参考文献:名称:Phenytoin therapy摘要:本发明揭示了一种治疗性组合物、一种剂型和一种方法,用于给予苯妥英,以治疗癫痫。公开号:US06346270B1
文献信息
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
[EN] COMPOUNDS AND THEIR USE AS BACE INHIBITORS<br/>[FR] COMPOSÉS ET LEUR UTILISATION EN TANT QU'INHIBITEURS DE BACE申请人:ASTRAZENECA AB公开号:WO2016055858A1公开(公告)日:2016-04-14The present application relates to compounds of formula (I), (la), or (lb) and their pharmaceutical compositions/preparations. This application further relates to methods of treating or preventing Αβ-related pathologies such as Down's syndrome, β- amyloid angiopathy such as but not limited to cerebral amyloid angiopathy or hereditary cerebral hemorrhage, disorders associated with cognitive impairment such as but not limited to MCI ("mild cognitive impairment"), Alzheimer's disease, memory loss, attention deficit symptoms associated with Alzheimer's disease, neurodegeneration associated with diseases such as Alzheimer's disease or dementia, including dementia of mixed vascular and degenerative origin, pre-senile dementia, senile dementia and dementia associated with Parkinson's disease.本申请涉及式(I)、(Ia)或(Ib)的化合物及其药物组合物/制剂。本申请进一步涉及治疗或预防与Αβ相关的病理学,如唐氏综合症,β-淀粉样蛋白血管病,如但不限于脑淀粉样蛋白血管病或遗传性脑出血,与认知损害相关的疾病,如但不限于MCI(“轻度认知损害”),阿尔茨海默病,记忆丧失,与阿尔茨海默病相关的注意力缺陷症状,与疾病如阿尔茨海默病或痴呆症相关的神经退行性疾病,包括混合性血管性和退行性起源的痴呆,早老性痴呆,老年性痴呆和与帕金森病相关的痴呆的方法。
-
[EN] METALLOENZYME INHIBITOR COMPOUNDS<br/>[FR] COMPOSÉS INHIBITEURS DE MÉTALLOENZYMES
-
4' SUBSTITUTED COMPOUNDS HAVING 5-HT6 RECEPTOR AFFINITY申请人:Dunn Robert公开号:US20080318941A1公开(公告)日:2008-12-25The present disclosure provides compounds having affinity for the 5-HT 6 receptor which are of the formula (I): wherein R 1 , R 2 , R 5 , R 6 , B, D, E, G, Q, x and n are as defined herein. The disclosure also relates to methods of preparing such compounds, compositions containing such compounds, and methods of use thereof.本公开提供了具有亲和力的化合物,其对5-HT 6 受体具有亲和力,其化学式为(I): 其中R1、R2、R5、R6、B、D、E、G、Q、x和n如本文所定义。本公开还涉及制备这种化合物的方法、含有这种化合物的组合物以及使用这些化合物的方法。
-
HETEROBICYCLIC COMPOUNDS申请人:Amgen Inc.公开号:US20130225552A1公开(公告)日:2013-08-29Heterobicyclic compounds of Formula (I): or a pharmaceutically-acceptable salt, tautomer, or stereoisomer thereof, as defined in the specification, and compositions containing them, and processes for preparing such compounds. Provided herein also are methods of treating disorders or diseases treatable by inhibition of PDE10, such as obesity, non-insulin dependent diabetes, schizophrenia, bipolar disorder, obsessive-compulsive disorder, Huntington's Disease, and the like.
表征谱图
-
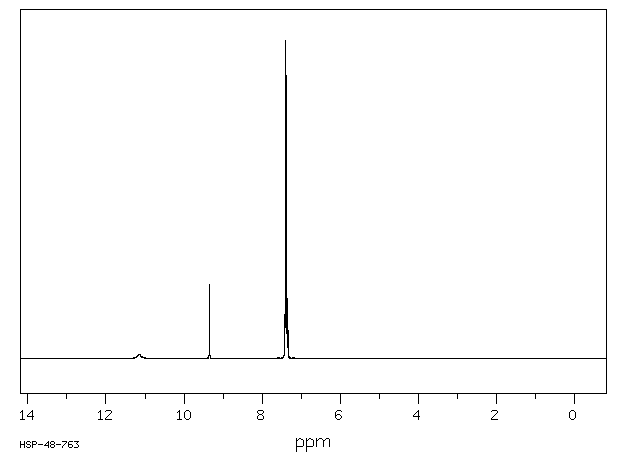
氢谱1HNMR
-
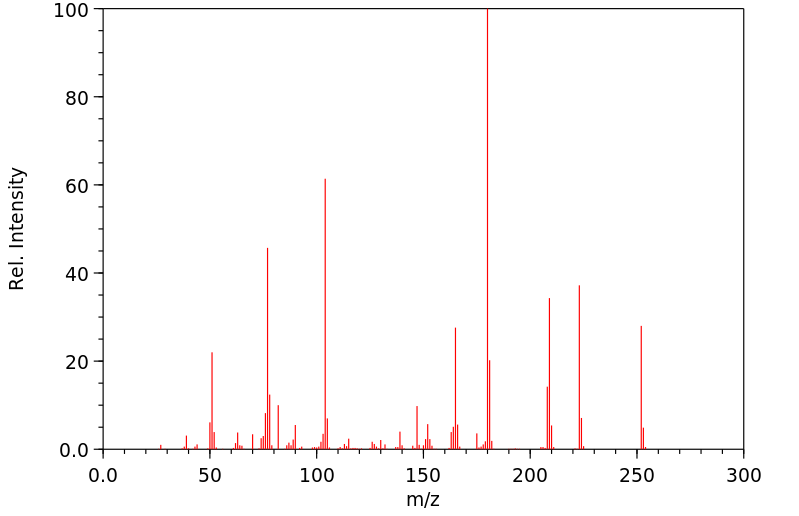
质谱MS
-
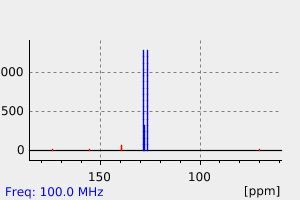
碳谱13CNMR
-
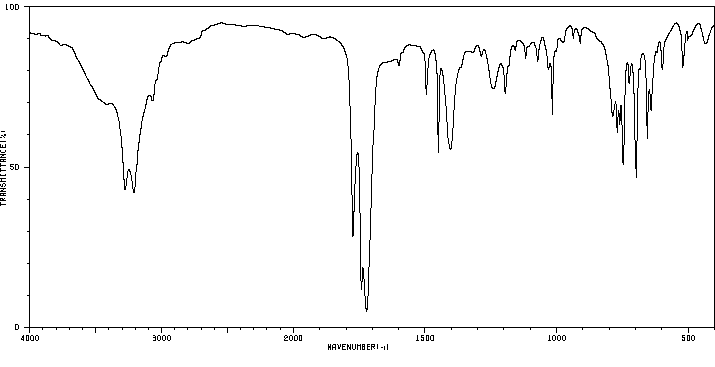
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-4-异丙基-2-恶唑烷硫酮
麻黄恶碱
顺-八氢-2H-苯并咪唑-2-酮
顺-1-(4-氟苯基)-4-[1-(4-氟苯基)-4-羰基-1,3,8-三氮杂螺[4.5]癸-8-基]环己甲腈
非达司他
降冰片烯缩醛3-((1S,2S,4S)-双环[2.2.1]庚-5-烯-2-羰基)恶唑烷-2-酮
阿齐利特
阿那昔酮
阿洛双酮
阿帕鲁胺
阿帕他胺杂质2
铟烷-2-YL-甲基胺盐酸
钾3-{2-[3-氰基-3-(十二烷基磺酰基)-2-丙烯-1-亚基]-1,3-噻唑烷-3-基}-1-丙烷磺酸酯
钠2-{[4,5-二羟基-3-(羟基甲基)-2-氧代-1-咪唑烷基]甲氧基}乙烷磺酸酯
重氮烷基脲
詹氏催化剂
解草恶唑
解草噁唑
表告依春
螺莫司汀
螺立林
螺海因氮丙啶
螺[咪唑烷-4,3'-吲哚啉]-2,2',5-三酮
螺[1-氮杂双环[2.2.2]辛烷-8,5'-咪唑烷]-2',4'-二酮
苯甲酸,4-氟-,2-[5,7-二(三氟甲基)-1,8-二氮杂萘-2-基]-2-甲基酰肼
苯氰二硫酸,1-氰基-1-甲基-4-氧代-4-(2-硫代-3-噻唑烷基)丁酯
苯妥英钠杂质8
苯妥英钠
苯妥英-D10
苯妥英
苯基硫代海因半胱氨酸钠盐
苯基硫代乙内酰脲-谷氨酸
苯基硫代乙内酰脲-蛋氨酸
苯基硫代乙内酰脲-苯丙氨酸
苯基硫代乙内酰脲-色氨酸
苯基硫代乙内酰脲-脯氨酸
苯基硫代乙内酰脲-缬氨酸
苯基硫代乙内酰脲-异亮氨酸
苯基硫代乙内酰脲-天冬氨酸
苯基硫代乙内酰脲-亮氨酸
苯基硫代乙内酰脲-丙氨酸
苯基硫代乙内酰脲-D-苏氨酸
苯基硫代乙内酰脲-(NΕ-苯基硫代氨基甲酰)-赖氨酸
苯基乙内酰脲-甘氨酸
苏氨酸-1-(苯基硫基)-2,4-咪唑烷二酮(1:1)
色氨酸标准品002
膦酸,(2-羰基-1-咪唑烷基)-,二(1-甲基乙基)酯
脱氢-1,3-二甲基尿囊素
脱氢-1,3,8-三甲基尿囊素
聚(d(A-T)铯)