(R)-4-异丙基-2-恶唑烷硫酮 | 1217463-35-8
中文名称
(R)-4-异丙基-2-恶唑烷硫酮
中文别名
——
英文名称
(4R)-4-isopropyl-1,3-oxazolidine-2-thione
英文别名
(R)-4-Isopropyloxazolidine-2-thione;(4R)-4-propan-2-yl-1,3-oxazolidine-2-thione
CAS
1217463-35-8
化学式
C6H11NOS
mdl
——
分子量
145.225
InChiKey
CIRDXQWBLPPFPN-YFKPBYRVSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:48-52 °C
-
沸点:171.2±23.0 °C(Predicted)
-
密度:1.11±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.83
-
拓扑面积:53.4
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险性防范说明:P261,P264,P270,P271,P280,P301+P312,P302+P352,P304+P340,P330,P363,P501
-
危险性描述:H302,H312,H332
反应信息
-
作为反应物:描述:甲氧基-三氟甲基苯 、 (R)-4-异丙基-2-恶唑烷硫酮 在 吡啶 作用下, 以 四氯化碳 为溶剂, 反应 15.0h, 生成 (R)-3,3,3-Trifluoro-1-((S)-4-isopropyl-2-thioxo-oxazolidin-3-yl)-2-methoxy-2-phenyl-propan-1-one参考文献:名称:Nagao, Yoshimitsu; Kumagai, Toshio; Yamada, Shozo, Journal of the Chemical Society. Perkin transactions I, 1985, p. 2361 - 2368摘要:DOI:
-
作为产物:描述:参考文献:名称:通过手性环骨架的硒重排反应,由N-酰基恶唑烷酮合成手性亚硒啉摘要:据报道,通过手性环状骨架的硒化重排,从容易获得的N-酰基恶唑烷二酮合成手性亚硒啉的途径。反应在元素硒,氢氯硅烷和胺的存在下进行。尽管所获得的亚硒唑啉产品的稳定性较低,但是成功制备了多种亚硒唑啉。DOI:10.1021/acs.orglett.8b02520
表征谱图
-
氢谱1HNMR
-
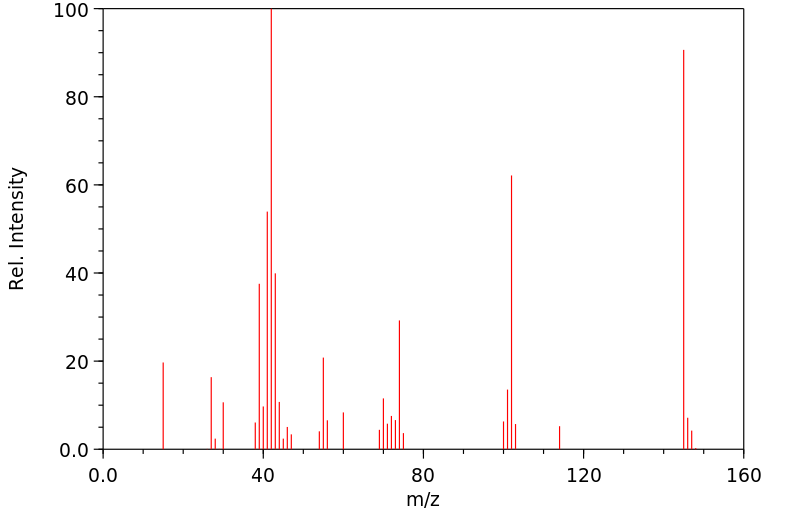
质谱MS
-
碳谱13CNMR
-
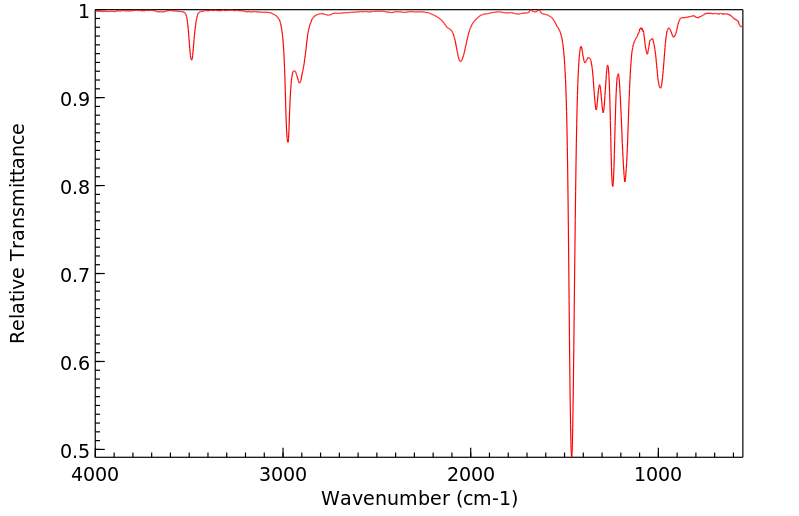
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-4-异丙基-2-恶唑烷硫酮
麻黄恶碱
顺-八氢-2H-苯并咪唑-2-酮
顺-1-(4-氟苯基)-4-[1-(4-氟苯基)-4-羰基-1,3,8-三氮杂螺[4.5]癸-8-基]环己甲腈
非达司他
降冰片烯缩醛3-((1S,2S,4S)-双环[2.2.1]庚-5-烯-2-羰基)恶唑烷-2-酮
阿齐利特
阿那昔酮
阿洛双酮
阿帕鲁胺
阿帕他胺杂质2
铟烷-2-YL-甲基胺盐酸
钾3-{2-[3-氰基-3-(十二烷基磺酰基)-2-丙烯-1-亚基]-1,3-噻唑烷-3-基}-1-丙烷磺酸酯
钠2-{[4,5-二羟基-3-(羟基甲基)-2-氧代-1-咪唑烷基]甲氧基}乙烷磺酸酯
重氮烷基脲
詹氏催化剂
解草恶唑
解草噁唑
表告依春
螺莫司汀
螺立林
螺海因氮丙啶
螺[咪唑烷-4,3'-吲哚啉]-2,2',5-三酮
螺[1-氮杂双环[2.2.2]辛烷-8,5'-咪唑烷]-2',4'-二酮
苯甲酸,4-氟-,2-[5,7-二(三氟甲基)-1,8-二氮杂萘-2-基]-2-甲基酰肼
苯氰二硫酸,1-氰基-1-甲基-4-氧代-4-(2-硫代-3-噻唑烷基)丁酯
苯妥英钠杂质8
苯妥英钠
苯妥英-D10
苯妥英
苯基硫代海因半胱氨酸钠盐
苯基硫代乙内酰脲-谷氨酸
苯基硫代乙内酰脲-蛋氨酸
苯基硫代乙内酰脲-苯丙氨酸
苯基硫代乙内酰脲-色氨酸
苯基硫代乙内酰脲-脯氨酸
苯基硫代乙内酰脲-缬氨酸
苯基硫代乙内酰脲-异亮氨酸
苯基硫代乙内酰脲-天冬氨酸
苯基硫代乙内酰脲-亮氨酸
苯基硫代乙内酰脲-丙氨酸
苯基硫代乙内酰脲-D-苏氨酸
苯基硫代乙内酰脲-(NΕ-苯基硫代氨基甲酰)-赖氨酸
苯基乙内酰脲-甘氨酸
苏氨酸-1-(苯基硫基)-2,4-咪唑烷二酮(1:1)
色氨酸标准品002
膦酸,(2-羰基-1-咪唑烷基)-,二(1-甲基乙基)酯
脱氢-1,3-二甲基尿囊素
脱氢-1,3,8-三甲基尿囊素
聚(d(A-T)铯)