5-氯氧化吲哚 | 17630-75-0
中文名称
5-氯氧化吲哚
中文别名
5-氯吲哚啉-2-酮;5-氯二氢吲哚-2-酮;5-氯羟吲哚;5-氯羟基吲哚;5-氯吲哚-2-酮;5-氯吲哚酮;5-氯-2-吲哚酮;5-氯-吲哚酮
英文名称
5-chloro-indolin-2-one
英文别名
5-Chlorooxindole;5-chloro-2-indolinone;5-chloro-1,3-dihydroindol-2-one
CAS
17630-75-0
化学式
C8H6ClNO
mdl
MFCD00191927
分子量
167.595
InChiKey
WWJLCYHYLZZXBE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:194-197 °C (lit.)
-
沸点:348.3±42.0 °C(Predicted)
-
密度:1.362±0.06 g/cm3(Predicted)
-
溶解度:微溶(2.0g/L)(25°C)。
-
稳定性/保质期:
常温常压下稳定,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:11
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:29.1
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险品标志:Xn
-
安全说明:S22,S24,S36/37,S36/37/39,S45,S61
-
危险类别码:R22,R62,R36/37/38,R43
-
WGK Germany:2
-
海关编码:2933790090
-
危险品运输编号:NONH for all modes of transport
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:常温密闭避光,通风干燥
SDS
| Name: | 5-Chlorooxindole Material Safety Data Sheet |
| Synonym: | |
| CAS: | 17630-75-0 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 17630-75-0 | 5-Chlorooxindole | unlisted |
Risk Phrases: 22 36/37/38 43 62
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful if swallowed. Irritating to eyes, respiratory system and skin. May cause sensitization by skin contact. Possible risk of impaired fertility.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
May cause sensitization by skin contact.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 17630-75-0: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: off-white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 194 - 197 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H6ClNO
Molecular Weight: 167.59
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 17630-75-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
5-Chlorooxindole - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 22 Harmful if swallowed.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
R 43 May cause sensitization by skin contact.
R 62 Possible risk of impaired fertility.
Safety Phrases:
S 24 Avoid contact with skin.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 17630-75-0: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 17630-75-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 17630-75-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-吲哚酮 2-oxoindole 59-48-3 C8H7NO 133.15 5-氯靛红 5-chloroindole 2,3-dione 17630-76-1 C8H4ClNO2 181.578 —— 5-chloro-3-imino indolin-2-one —— C8H5ClN2O 180.593 5-氯-3-重氮-1,3-二氢-2H-吲哚-2-酮 5-chloro-3-hydrazonoindolin-2-one 100487-78-3 C8H6ClN3O 195.608 3,3-二溴-5-氯-1,3-二氢吲哚-2-酮 3,3-Dibromo-5-chloroindol-2(3H)-one 113423-48-6 C8H4Br2ClNO 325.387 1-羟基-3H-吲哚-2-酮 1,3-dihydro-1-hydroxyindole-2-one 18108-55-9 C8H7NO2 149.149 —— 1-methoxyindolin-2-one 65816-14-0 C9H9NO2 163.176 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 5-chloro-1-methylindolin-2-one 41192-33-0 C9H8ClNO 181.622 5-氯-1,3-二氢-3-甲基-2H-吲哚-2-酮 5-chloro-3-methylindolin-2-one 90537-20-5 C9H8ClNO 181.622 5-氯靛红 5-chloroindole 2,3-dione 17630-76-1 C8H4ClNO2 181.578 —— 5-chloro-3-methylene-2-oxindole 442883-74-1 C9H6ClNO 179.606 —— 5-chloro-1-ethyl-2-indolinone 41192-34-1 C10H10ClNO 195.648 7-溴-5-氯吲哚啉-2-酮 7-bromo-5-chloroindolin-2-one 215433-19-5 C8H5BrClNO 246.491 —— 5-chloro-1-hydroxymethyl-1,3-dihydro-indol-2-one 854141-75-6 C9H8ClNO2 197.621 —— 1-acetyl-5-chloroindolin-2-one 103585-68-8 C10H8ClNO2 209.632 5-氯-1,3-二氢吲哚-2-硫酮 5-chloroindoline-2-thione 73424-95-0 C8H6ClNS 183.661 5-氯-1H-吲哚-2,3-二酮 3-肟 5-chloro-3-(hydroxyimino)indolin-2-one 85124-16-9 C8H5ClN2O2 196.593 3,3-二溴-5-氯-1,3-二氢吲哚-2-酮 3,3-Dibromo-5-chloroindol-2(3H)-one 113423-48-6 C8H4Br2ClNO 325.387 —— 5-chloro-3-isopropylindolin-2-one 1225922-11-1 C11H12ClNO 209.675 —— (5-chloro-2-oxo-2,3-dihydroindol-1-yl)acetic acid 771533-13-2 C10H8ClNO3 225.631 5-氯-2-氧化吲哚-1-甲酰胺 5-chloro-2,3-dihydro-2-oxo-1H-indole-1-carboxamide 100599-06-2 C9H7ClN2O2 210.62 —— 5-chloro-3-(propan-2-ylidene)indolin-2-one 1225386-83-3 C11H10ClNO 207.659 —— 3-benzyl-5-chloroindolin-2-one 1165901-92-7 C15H12ClNO 257.719 —— 5-Chloro-3-(dimethylaminomethylene)-2(1H,3H)-indolone 52508-95-9 C11H11ClN2O 222.674 —— 5-Chlor-3-dimethylaminomethylenoxindol 52508-95-9 C11H11ClN2O 222.674 —— (E)-3-benzylidene-5-chloroindolin-2-one 902757-75-9 C15H10ClNO 255.703 —— 3-benzylidene-5-chloroindolin-2-one —— C15H10ClNO 255.703 —— 5-chloro-1-methoxycarbonyl-2-oxo-2,3-dihydroindole 197776-09-3 C10H8ClNO3 225.631 —— 5-chloro-3-(4-hydroxybenzylidene)indolin-2-one 387342-90-7 C15H10ClNO2 271.703 —— 2-(2-amino-5-chlorophenyl)acetic acid 500572-08-7 C8H8ClNO2 185.61 —— 2-(5-chloro-2-oxoindolin-3-ylidene)malononitrile 202288-38-8 C11H4ClN3O 229.625 —— 7-Benzoyl-5-chlorindolin-2-on 61085-27-6 C15H10ClNO2 271.703 —— 5-chloro-1-ethoxycarbonyl-2-oxo-2,3-dihydroindole 197776-07-1 C11H10ClNO3 239.658 —— 5-chloro-3-(4-methoxybenzyliden)indolin-2-one —— C16H12ClNO2 285.73 —— 5-chloro-3-(piperidin-4-yl)indolin-2-one 1368792-02-2 C13H15ClN2O 250.728 —— 1-[4-[(Z)-(5-chloro-2-oxo-1H-indol-3-ylidene)methyl]phenyl]-3-phenylurea 946618-62-8 C22H16ClN3O2 389.841 —— (E)-5-chloro-3-(4-trifluoromethylbenzylidene)-1,3-dihydro-indol-2-one 1421506-30-0 C16H9ClF3NO 323.702 —— 5'-chlorospiro[cyclopropane-1,3'-indoline] —— C10H10ClN 179.649 —— (E) 3-(5-chloro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-benzonitrile 1421506-40-2 C16H9ClN2O 280.713 —— 5-chloro-3-[(4-phenylphenyl)methylidene]-1H-indol-2-one —— C21H14ClNO 331.801 5-溴靛红 5-Bromo-1H-indole-2,3-dione 87-48-9 C8H4BrNO2 226.029 —— 3-(4-butoxybenzyliden)-5-chloroindolin-2-one 387343-25-1 C19H18ClNO2 327.81 —— N-[3-(dimethylamino)propyl]-5-chloro-2,3-dihydro-2-oxo-1H-indole-1-carboxamide 265994-71-6 C14H18ClN3O2 295.769 —— 5-chloro-3-(4-(3-chloropropoxy)benzyliden)indolin-2-one 1227623-26-8 C18H15Cl2NO2 348.229 —— (E)-5-chloro-3-(3,4-difluoro-benzylidene)-1,3-dihydro-indol-2-one 1421506-33-3 C15H8ClF2NO 291.684 —— (E)-5-chloro-3-(3-methoxybenzylidene)-dihydro-indol-2-one 1148119-10-1 C16H12ClNO2 285.73 —— 5'-chloro-spiro[cyclohexane-1,3'-indolin]-2'-one 156232-14-3 C13H14ClNO 235.713 —— (E)-5-chloro-3-(2-fluoro-benzylidene)-1,3-dihydro-indol-2-one 1421506-21-9 C15H9ClFNO 273.694 —— 5-chloro-3-(2-fluorobenzylidene)indolin-2-one 387343-46-6 C15H9ClFNO 273.694 —— (3E)-5-chloro-3-[(4-morpholin-4-ylphenyl)methylidene]-1H-indol-2-one —— C19H17ClN2O2 340.809 —— 5-chloro-3-(4-morpholin-4-yl-benzylidene)-1,3-dihydroindol-2-one —— C19H17ClN2O2 340.809 —— 5-chloro-3-(4-(2-(dimethylamino)ethoxy)benzyliden)indolin-2-one 1415609-30-1 C19H19ClN2O2 342.825 —— 2-(4-((5-chloro-2-oxoindolin-3-ylidene)methyl)phenoxy)acetamide 930552-50-4 C17H13ClN2O3 328.755 —— (E)-5-chloro-3-(3-(trifluoromethyl)benzylidene)-1,3-dihydro-indol-2-one 1421506-29-7 C16H9ClF3NO 323.702 —— 5-chloro-3-[[3-(trifluoromethyl)phenyl]methylidene]-1H-indol-2-one —— C16H9ClF3NO 323.702 - 1
- 2
- 3
- 4
- 5
反应信息
-
作为反应物:描述:5-氯氧化吲哚 在 4-二甲氨基吡啶 、 ammonium carbonate 、 三乙胺 作用下, 以 四氢呋喃 、 二氯甲烷 、 N,N-二甲基甲酰胺 为溶剂, 反应 14.5h, 生成 替尼达普参考文献:名称:替尼达实用新合成摘要:描述了一种新的实用合成替尼达普的开发。5-氯-2-氧代-2,3-二氢吲哚的N,O-二烷氧基(芳氧基)羰基化,然后除去O-烷氧基(芳氧基)羰基得到1-[烷氧基(芳氧基)羰基]-5-氯-2-氧代-2,3-二氢吲哚的收率良好。后一种化合物在 3 位进行了酚化。讨论了 DMAP 在酰化反应中的作用。在溶液和固相中研究了 thenoylated 产物及其烯醇盐的结构。5-chloro-3-[1-hydroxy-1-(2-thienyl)methylene]-2-oxo-1-phenoxycarbonyl-2,3-dihydroindole 的氨解以高产率提供相应的 1-carbamoyl 衍生物 (tenidap)。相应的 1-乙氧基和 1-甲氧基羰基衍生物不能类似地转化为替尼达;DOI:10.1021/op990067a
-
作为产物:参考文献:名称:合成和5-抗肿瘤活性[1-(3-(二甲氨基)丙基)-5-卤代-2- oxoindolin-(3 Ž -亚基甲基)] -2,4-二甲基-1- ħ吡咯-3-甲酰胺摘要:我们在此报告基于已知的多靶点受体酪氨酸激酶抑制剂舒尼替尼和先前发现的化合物TMP-20的结构特征,设计和合成新型1- [3-(二甲基氨基)丙基]吲哚-2-酮衍生物在我们的实验室中具有良好的抗肿瘤活性。评价了这些新合成的衍生物对五种人类癌细胞系和VEGF / bFGF刺激的HUVEC的体外活性。结果显示,所有目标化合物1a – p均显示出强大的抗肿瘤活性,化合物1e – h(IC 50值:0.45–5.08μM)比舒尼替尼(IC 50值:1.35–6.61μM)更具活性,并且活性最高的化合物1h(IC50:0.47–3.11μM)对所有五种癌细胞系的效力比舒尼替尼高2.1–4.6倍。另外,与舒尼替尼一样,1a – p对bFGF刺激的HUVEC以外的VEGF刺激的HUVEC具有更高的选择性。DOI:10.1016/j.bmcl.2011.03.031
-
作为试剂:参考文献:名称:Anti-inflammatory methods using 2-amino-3-(5- and 6-)benzoylphenylacetic摘要:化合物的式子为:##STR1## 其中R为氢或低碳基,R.sup.1为氢、低碳基、钠或钾,R.sup.2为氢、卤素或低烷氧基,X为氢、低碳基、卤素、硝基或三氟甲基,Y为氢、低碳基、卤素、硝基或三氟甲基,Am为初级氨基(--NH.sub.2)、甲基氨基或二甲基氨基。这些化合物是通过将4-(5-和7-)苯甲酰吲哚烷-2-酮水解制备得到的2-氨基-3-(5-和6-)苯甲酰基乙酸,其中氨基为初级氨基。游离酸转化为酯和金属盐。本发明揭示了钙和镁盐。这些化合物具有抗炎活性,能有效降低高脂血症大鼠的胆固醇水平,并抑制血小板聚集。公开号:US04045576A1
文献信息
-
Kinase inhibitors申请人:Amgen Inc.公开号:US20040116388A1公开(公告)日:2004-06-17The invention relates to inhibitors of enzymes that bind to ATP or GTP and/or catalyze phosphoryl transfer, compositions comprising the inhibitors, and methods of using the inhibitors and inhibitor compositions. The inhibitors and compositions comprising them are useful for treating disease or disease symptoms. The invention also provides for methods of making phosphoryl transferase inhibitor compounds, methods of inhibiting phosphoryl transferase activity, and methods for treating disease or disease symptoms.
-
一种抑制IDO的化合物及其应用
-
Palladium-Catalyzed C–H Activation and Cyclization of Anilides with 2-Iodoacetates and 2-Iodobenzoates: An Efficient Method toward Oxindoles and Phenanthridones作者:Chien-Hong Cheng、Parthasarathy Gandeepan、Pachaiyappan RajamalliDOI:10.1055/s-0035-1561856日期:——Abstract A concise approach to the synthesis of oxindoles and phenanthridones from anilides is described. In the presence of catalytic amount of Pd(OAc)2, 2-iodoacetates and 2-iodobenzoates can be used to functionalize ortho C–H bond of anilides, which subsequently undergo intramolecular cyclization to give the products. A possible reaction mechanism that involves a PdII/PdIV catalytic cycle is proposed
-
A cinchona alkaloid catalyzed enantioselective sulfa-Michael/aldol cascade reaction of isoindigos: construction of chiral bispirooxindole tetrahydrothiophenes with vicinal quaternary spirocenters作者:Yong-Yuan Gui、Jian Yang、Liang-Wen Qi、Xiao Wang、Fang Tian、Xiao-Nian Li、Lin Peng、Li-Xin WangDOI:10.1039/c5ob00774g日期:——
Enantioselective sulfa-Michael/aldol reaction of isoindigos has been successfully developed to afford bispirooxindole tetrahydrothiophenes with vicinal quaternary spirocenters.
-
3-heteroarylidene-2-indolinone protein kinase inhibitors申请人:Sugen, Inc.公开号:US06486185B1公开(公告)日:2002-11-26The present invention relates to novel 3-heteroarylidene-2-indolinone compounds and physiologically acceptable salts thereof which modulate the activity of protein kinases and therefore are expected to be useful in the prevention and treatment of protein kinase related cellular disorders such as cancer.本发明涉及新颖的3-杂环亚烯基-2-吲哚酮化合物及其生理上可接受的盐,这些化合物调节蛋白激酶的活性,因此预计在预防和治疗蛋白激酶相关的细胞疾病,如癌症方面具有用处。
表征谱图
-
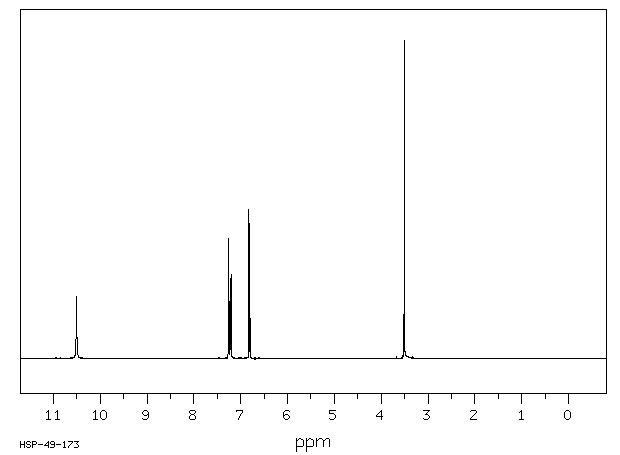
氢谱1HNMR
-
质谱MS
-
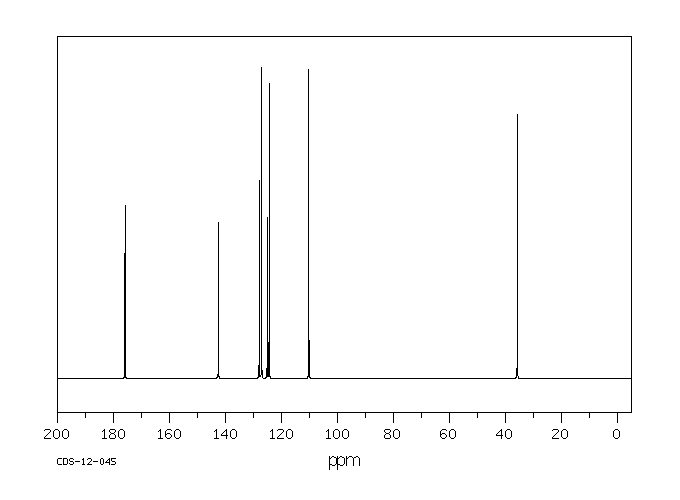
碳谱13CNMR
-
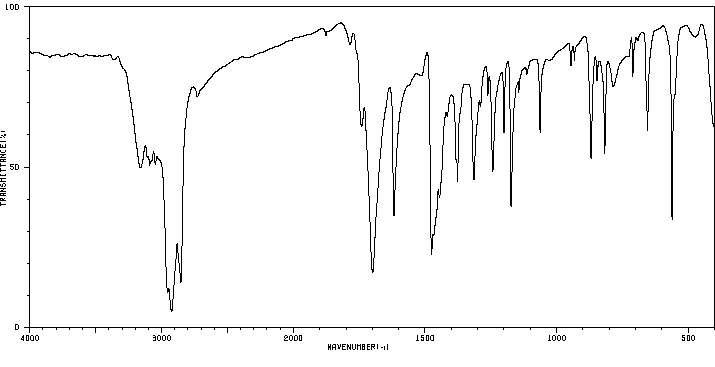
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Z)-3-[[[2,4-二甲基-3-(乙氧羰基)吡咯-5-基]亚甲基]吲哚-2--2-
(S)-(-)-5'-苄氧基苯基卡维地洛
(R)-(+)-5'-苄氧基卡维地洛
(R)-卡洛芬
(N-(Boc)-2-吲哚基)二甲基硅烷醇钠
(E)-2-氰基-3-(5-(2-辛基-7-(4-(对甲苯基)-1,2,3,3a,4,8b-六氢环戊[b]吲哚-7-基)-2H-苯并[d][1,2,3]三唑-4-基)噻吩-2-基)丙烯酸
(4aS,9bR)-6-溴-2,3,4,4a,5,9b-六氢-1H-吡啶并[4,3-B]吲哚
(3Z)-3-(1H-咪唑-5-基亚甲基)-5-甲氧基-1H-吲哚-2-酮
(3Z)-3-[[[4-(二甲基氨基)苯基]亚甲基]-1H-吲哚-2-酮
(3R)-(-)-3-(1-甲基吲哚-3-基)丁酸甲酯
(3-氯-4,5-二氢-1,2-恶唑-5-基)(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙酸
齐多美辛
鸭脚树叶碱
鸭脚木碱,鸡骨常山碱
鲜麦得新糖
高氯酸1,1’-二(十六烷基)-3,3,3’,3’-四甲基吲哚碳菁
马鲁司特
马鞭草(VERBENAOFFICINALIS)提取物
马来酸阿洛司琼
马来酸替加色罗
顺式-ent-他达拉非
顺式-1,3,4,4a,5,9b-六氢-2H-吡啶并[4,3-b]吲哚-2-甲酸乙酯
顺式-(+-)-3,4-二氢-8-氯-4'-甲基-4-(甲基氨基)-螺(苯并(cd)吲哚-5(1H),2'(5'H)-呋喃)-5'-酮
靛青二磺酸二钾盐
靛藍四磺酸
靛红联二甲酚
靛红磺酸钠
靛红磺酸
靛红乙烯硫代缩酮
靛红-7-甲酸甲酯
靛红-5-磺酸钠
靛红-5-磺酸
靛红-5-硫酸钠盐二水
靛红-5-甲酸甲酯
靛红
靛玉红衍生物E804
靛玉红3'-单肟5-磺酸
靛玉红-3'-单肟
靛玉红
靛噻
青色素3联己酸染料,钾盐
雷马曲班
雷莫司琼杂质13
雷莫司琼杂质12
雷莫司琼杂质
雷替尼卜定
雄甾-1,4-二烯-3,17-二酮
阿霉素的代谢产物盐酸盐
阿贝卡尔
阿西美辛杂质3