3-硝基氯苯 | 121-73-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:43-47 °C(lit.)
-
沸点:236 °C(lit.)
-
密度:1.534 g/mL at 25 °C(lit.)
-
闪点:218 °F
-
溶解度:加热时易溶于酒精。
-
LogP:2.49
-
物理描述:3-chloronitrobenzene appears as pale yellow crystals. Insoluble in water. (NTP, 1992)
-
颜色/状态:Pale-yellow orthorhombic prisms from alcohol
-
蒸汽密度:Relative vapor density (air = 1): 5.44
-
蒸汽压力:0.097 mm Hg at 25 °C
-
亨利常数:1.35e-05 atm-m3/mole
-
自燃温度:500 °F (260 °C)
-
燃烧热:-2.82E+9 J/kmol
-
汽化热:5.58E+7 J/kmol at melting point at 317.65 deg K
-
表面张力:4.37X10-2 N/m at 317.65 deg K
-
折光率:Index of refraction: 1.5374 at 80 °C, alpha
-
保留指数:1179;1204.6;1185
-
稳定性/保质期:
-
稳定性:稳定。
-
禁配物:强氧化剂、强碱、强还原剂。
-
避免接触的条件:受热。
-
聚合危害:不聚合。
-
分解产物:氮氧化物、氯化氢。
-
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:45.8
-
氢给体数:0
-
氢受体数:2
ADMET
安全信息
-
TSCA:Yes
-
危险等级:6.1
-
安全说明:S22,S24/25,S28A,S36/37/39,S38,S45,S60,S61
-
危险品运输编号:UN 1578 6.1/PG 2
-
WGK Germany:2
-
海关编码:29049085
-
危险类别:6.1
-
危险品标志:T
-
危险类别码:R23/24/25
-
RTECS号:CZ0940000
-
包装等级:II
-
储存条件:储存注意事项: - 储存于阴凉、通风的库房。 - 远离火种、热源。 - 包装需密封。 - 应与氧化剂、还原剂、碱类及食用化学品分开存放,切忌混储。 - 配备相应品种和数量的消防器材。 - 储区应备有合适的材料以收容泄漏物。
制备方法与用途
间氯硝基苯为浅黄色斜方棱晶。微溶于水,可溶于乙醇、乙醚、苯等大多数有机溶剂。还原时生成间氨基氯苯。该化合物由硝基苯在碘存在下氯化而制得。
用途主要用于制造间氯苯胺、偶氮染料、颜料、药物及杀虫剂等。此外,它也是有机合成原料和染料中间体,广泛用于制备间氯苯胺和间二氯苯等化合物。在生化研究中也具有重要应用价值。
生产方法-
由硝基苯与铁屑在氯化塔中反应而得。具体步骤为:加入干燥的硝基苯及铁屑,通入氯气,并将温度控制在40-45℃之间。当相对密度达到1.35(25℃)、凝固点为23℃时停止通氯。之后用压缩空气吹除氯及氯化氢约3小时,再用水洗至中性,进行减压分馏,切取凝固点高于22℃的馏分为结晶液。冷却后保持13℃温度2小时,随后升温至43℃,使结晶熔解以获得最终产品。
- 有毒物品
- 毒性分级:高毒
- 急性毒性:
- 大鼠口服LD50:420毫克/公斤
- 小鼠口服LD50:380毫克/公斤
- 可燃性危险特性:遇明火可燃;燃烧会产生有毒的氯化物和氮氧化物烟雾
- 储运特性:
- 库房需保持通风、低温干燥环境,与氧化剂及食品添加剂分开存放
- 时间加权平均容许浓度(TWA):1毫克/立方米
- 短时间接触容许浓度(STEL):2毫克/立方米
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-氯-4-硝基苯胺 2-Chloro-4-nitroaniline 121-87-9 C6H5ClN2O2 172.571 4-氯-2-硝基苯胺 4-Chloro-2-nitroaniline 89-63-4 C6H5ClN2O2 172.571 1-氯-3-亚硝基苯 1-chloro-3-nitrosobenzene 932-78-5 C6H4ClNO 141.557 2,3-二氯硝基苯 1,2-Dichloro-3-nitrobenzene 3209-22-1 C6H3Cl2NO2 192.001 2-氯-6-硝基苯胺 2-chloro-6-nitroaniline 769-11-9 C6H5ClN2O2 172.571 对硝基氯苯 4-chlorobenzonitrile 100-00-5 C6H4ClNO2 157.556 4-氯-2-硝基甲苯 4-chloro-2-nitrotoluene 89-59-8 C7H6ClNO2 171.583 硝基苯 nitrobenzene 98-95-3 C6H5NO2 123.111 4-氯-2-硝基溴苄 4-chloro-2-nitrobenzyl bromide 52311-59-8 C7H5BrClNO2 250.479 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-氯-4-硝基苯胺 2-Chloro-4-nitroaniline 121-87-9 C6H5ClN2O2 172.571 3,4-二氯硝基苯 3,4-dichloronitrobenzene 99-54-7 C6H3Cl2NO2 192.001 2,5-二氯硝基苯 2,5-dichloronitrobenzene 89-61-2 C6H3Cl2NO2 192.001 4-氯-2-硝基苯胺 4-Chloro-2-nitroaniline 89-63-4 C6H5ClN2O2 172.571 1-氯-3-亚硝基苯 1-chloro-3-nitrosobenzene 932-78-5 C6H4ClNO 141.557 2-氯-1-碘-4-硝基苯 3-chloro-4-iodonitrobenzene 41252-96-4 C6H3ClINO2 283.453 2-氯-4-硝基苯酚 2-chloro-4-nitrophenol 619-08-9 C6H4ClNO3 173.556 3-氯苯基羟胺 N-(3-chlorophenyl)hydroxylamine 10468-17-4 C6H6ClNO 143.573 2,3-二氯硝基苯 1,2-Dichloro-3-nitrobenzene 3209-22-1 C6H3Cl2NO2 192.001 2-氯-6-硝基苯胺 2-chloro-6-nitroaniline 769-11-9 C6H5ClN2O2 172.571 4-氯-2-硝基苯酚 p-chloro-o-nitrophenol 89-64-5 C6H4ClNO3 173.556 3,4-二硝基氯苯 3,4-dinitro-chlorobenzene 610-40-2 C6H3ClN2O4 202.554 2-氯-6-硝基苯酚 2-chloro-6-nitrophenol 603-86-1 C6H4ClNO3 173.556 —— 2-chloro-N-ethyl-4-nitroaniline 6085-93-4 C8H9ClN2O2 200.625 硝基苯 nitrobenzene 98-95-3 C6H5NO2 123.111 1-氯-2-碘-3-硝基苯 1-chloro-2-iodo-3-nitrobenzene 32337-97-6 C6H3ClINO2 283.453 2,6-二氯硝基苯 2,6-dichloronitrobenzene 601-88-7 C6H3Cl2NO2 192.001 2-溴-3-氯硝基苯 2-bromo-1-chloro-3-nitrobenzene 19128-48-4 C6H3BrClNO2 236.452 —— 2-chloro-4-nitro-N-phenylaniline 16611-23-7 C12H9ClN2O2 248.669 2,4-二氯硝基苯 2,4-dichloronitrobenzene 611-06-3 C6H3Cl2NO2 192.001 (2-氯-4-硝基苯基)-乙腈 (2-Chloro-4-nitrophenyl)acetonitrile 89277-99-6 C8H5ClN2O2 196.593 2-氯-1-乙氧基-4-硝基苯 2-chloro-1-ethoxy-4-nitrobenzene 5493-71-0 C8H8ClNO3 201.609 - 1
- 2
- 3
反应信息
-
作为反应物:参考文献:名称:DE513798摘要:公开号:
-
作为产物:描述:参考文献:名称:二甲基亚砜在四氢呋喃中用 t-BuONO 加速芳香胺的还原脱氨基摘要:已经实现了通过一锅还原脱氨基将芳胺转化为芳烃的有效方法。发现在四氢呋喃中使用 t-BuONO 可以通过二甲基亚砜加速还原脱氨基,并在温和条件下提供具有良好产率的脱氨基产物。讨论了一个合理的机制。DOI:10.3184/174751918x15402967122774
-
作为试剂:参考文献:名称:Synthesis of methyl-N-arylcarbamates by the carbonylation of azoxy, azo, and nitro compounds摘要:DOI:10.1007/bf00995693
文献信息
-
Cobalt nanoclusters coated with N-doped carbon for chemoselective nitroarene hydrogenation and tandem reactions in water作者:Silvia Gutiérrez-Tarriño、Sergio Rojas-Buzo、Christian W. Lopes、Giovanni Agostini、Jose. J. Calvino、Avelino Corma、Pascual Oña-BurgosDOI:10.1039/d1gc00706h日期:——selective non-noble metal-based catalysts for the chemoselective reduction of nitro compounds in aquo media under mild conditions is an attractive research area. Herein, the synthesis of subnanometric and stable cobalt nanoclusters, covered by N-doped carbon layers as core–shell (Co@NC-800), for the chemoselective reduction of nitroarenes is reported. The Co@NC-800 catalyst was prepared by the pyrolysis用于在温和条件下化学选择性还原水介质中硝基化合物的活性和选择性非贵金属基催化剂的开发是一个有吸引力的研究领域。在此,报道了合成亚纳米和稳定的钴纳米团簇,由 N 掺杂的碳层作为核 - 壳层(Co@NC-800)覆盖,用于硝基芳烃的化学选择性还原。所述钴@ NC-800催化剂是由钴(TPY)的热解制备的2复合浸渍在 Vulcan 碳上。事实上,基于六个 N-Co 键的分子复合物的使用推动了由 N 掺杂碳层覆盖的明确和分布的钴核-壳纳米簇的形成。为了阐明它的性质,它已经通过使用几种先进的技术来充分表征。此外,这种制备的催化剂在温和的反应条件下对用H 2还原硝基化合物显示出高活性、化学选择性和稳定性。水被用作绿色溶剂,改善了之前基于钴催化剂的结果。此外,Co@NC-800通过硝基芳烃的还原胺化,该催化剂对于一锅合成仲芳基胺和异吲哚啉酮也具有活性和选择性。最后,基于衍射和光谱研究,已提出具有表面 CoN
-
Highly chemoselective reduction of nitroarenes over non-noble metal nickel-molybdenum oxide catalysts作者:Haigen Huang、Xueguang Wang、Xu Li、Chenju Chen、Xiujing Zou、Weizhong Ding、Xionggang LuDOI:10.1039/c6gc03141b日期:——Chemoselective reduction of nitroarenes is an important transformation for the production of arylamines, which are the primary intermediates in the synthesis of pharmaceuticals, agrochemicals and dyes. Heterogeneous non-noble metal nickel-molybdenum...
-
Recyclable and Selective Nitroarene Hydrogenation Catalysts Based on Carbon-Coated Cobalt Oxide Nanoparticles作者:Bingfeng Chen、Fengbo Li、Zhijun Huang、Guoqing YuanDOI:10.1002/cctc.201501265日期:2016.3.18through direct heating treatment of cobalt oxide precursors incipiently deposited over nanographite materials. Cobalt oxides are partially reduced to active zero‐valent metal species and the simultaneous formation of carbon layers over the nanoparticles protects them from oxidation and deactivation. This nanocatalyst performs excellently in chemoselective hydrogenation of some challenging nitroarenes with
-
Copper-Based Intermetallic Electride Catalyst for Chemoselective Hydrogenation Reactions作者:Tian-Nan Ye、Yangfan Lu、Jiang Li、Takuya Nakao、Hongsheng Yang、Tomofumi Tada、Masaaki Kitano、Hideo HosonoDOI:10.1021/jacs.7b08252日期:2017.11.29The development of transition metal intermetallic compounds, in which active sites are incorporated in lattice frameworks, has great potential for modulating the local structure and the electronic properties of active sites, and enhancing the catalytic activity and stability. Here we report that a new copper-based intermetallic electride catalyst, LaCu0.67Si1.33, in which Cu sites activated by anionic过渡金属间化合物的开发,其中活性位点并入晶格骨架中,具有很大的潜力来调节活性位点的局部结构和电子性质,并增强催化活性和稳定性。在这里,我们报道了一种新型的铜基金属间电催化剂LaCu 0.67 Si 1.33,其中具有低功函的阴离子电子激活的Cu位原子原子地分散在晶格骨架中,并提供硝基芳烃的选择性加氢,其营业额高40倍以上频率(TOF高达5084 h –1),而不是经过深入研究的金属负载催化剂。利用同位素效应的动力学分析表明,氢键的裂解是决定速率的步骤。出乎意料的是,LaCu 0.67 Si 1.33的高载流子密度和低逸出功(LWF)特性使得能够以极低的活化能(E a = 14.8 kJ·mol –1)活化氢分子。此外,LaCu 0.67 Si 1.33的高氧亲合力可实现通过硝基优先吸附硝基芳烃表面,导致高化学选择性。本发明的有效催化剂可以进一步引发具有高活性的其他含氧官能团例如醛和酮的氢化
-
Microwave-Assisted Rapid and efficient Reduction of Aromatic Nitro Compounds to Amines with Propan-2-ol over Nanosized Perovskite-type SmFeO<sub>3</sub> powder as a New Recyclable Heterogeneous Catalyst作者:Saeid Farhadi、Firouzeh Siadatnasab、Maryam KazemDOI:10.3184/174751911x12964930076647日期:2011.2
Nanosized perovskite-type SmFeO3 powder, prepared through the thermal decomposition of Sm[Fe(CN)6].4H2O with an average particle diameter of 28 nm and a specific surface area of 42 m2 g−1, was used as a recyclable heterogeneous catalyst for the efficient and selective reduction of aromatic nitro compounds into the corresponding amines by using propan-2-ol as a hydrogen donor (reducing agent) and KOH as a promoter under microwave irradiation. This highly regio- and chemoselective catalytic method is fast, clean, inexpensive, high yielding and also compatible with the substrates containing easily reducible functional groups. In addition, the nanosized SmFeO3 catalyst can be reused without loss of activity.
表征谱图
-
氢谱1HNMR
-
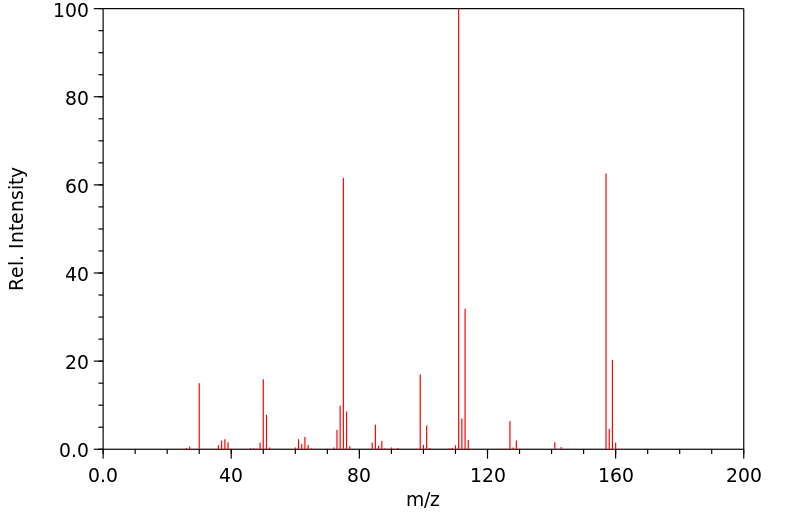
质谱MS
-
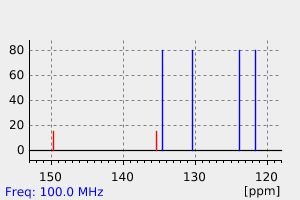
碳谱13CNMR
-
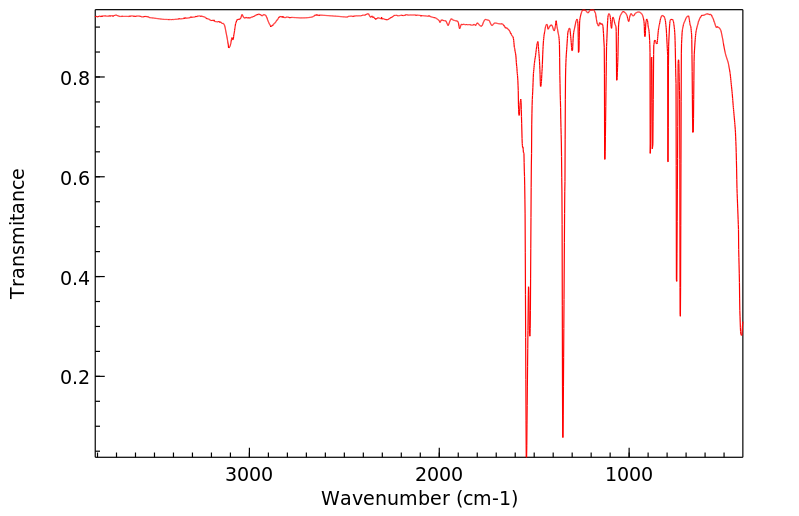
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息