甲基丙烯酸甲酯 | 80-62-6
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-48 °C (lit.)
-
沸点:100 °C (lit.)
-
密度:0.936 g/mL at 25 °C (lit.)
-
蒸气密度:3.5 (vs air)
-
闪点:50 °F
-
溶解度:15g/l
-
介电常数:2.9(20℃)
-
暴露限值:NIOSH REL: TWA 100 ppm (410 mg/m3), IDLH 1,000 ppm; OSHA PEL: TWA 100 ppm; ACGIH TLV: TWA 100 ppm with intended TWA and STEL values of 50 and 100 ppm, respectively.
-
LogP:1.38 at 20℃
-
物理描述:Methyl methacrylate monomer appears as a clear colorless liquid. Slightly soluble in water and floats on water. Vapors heavier than air. Vapors irritate the eyes and respiratory system. Containers must be heavily insulated or shipped under refrigeration. An inhibitor such as hydroquinone, hydroquinone methyl ester and dimethyl t-butylphenol is added to keep the chemical from initiating polymerization. The chemical may polymerize exothermically if heated or contaminated with strong acid or base. If the polymerization takes place inside a container, the container may rupture violently. Used to make plastics.
-
颜色/状态:Colorless volatile liquid
-
气味:Characteristic quality: sulfur-like, sweet, sharp; hedonic tone: unpleasant
-
蒸汽密度:3.45 (NTP, 1992) (Relative to Air)
-
蒸汽压力:38.5 mm Hg at 25 °C
-
大气OH速率常数:2.60e-11 cm3/molecule*sec
-
自燃温度:815 °F (435 °C)
-
分解:When heated to decomposition it emits acrid smoke and irritating fumes.
-
燃烧热:-11,400 BTU/lb = 6,310 cal/g = -264X10+5 J/kg (estimated)
-
汽化热:36.0 kJ/mol at 100.5 °C
-
表面张力:0.028 N/m at 20 °C.
-
电离电位:9.70 eV
-
聚合:The monomer tends to self-polymerize and this may become explosive ... Exposure of the purified (unstabilized) monomer to air at room temp for 2 months generated an ester-oxygen interpolymer, which exploded on evaporation of the surplus monomer at 60 °C (but not at 40 °C).
-
气味阈值:0.05 ppm
-
折光率:Index of refraction: 1.4142 at 20 °C
-
相对蒸发率:3.1 (Butyl acetate = 1)
-
保留指数:723;672;696;700;694;677;699;677;677;677;710;699;696;694.1;696.1;696;696;699;692.9;677;699
-
稳定性/保质期:
-
易挥发,易燃,能与空气形成爆炸性混合物。它溶于乙醇、乙醚、丙酮等多种有机溶剂,并微溶于乙二醇和水。受光、热及催化作用容易聚合,也可与其他单体共聚;由于含有双键和羧酸基团,还易进行加成、卤化、亲核取代以及酯交换反应。通常需要加入10-5%的氢醌单甲醚作为阻聚剂。
-
本品毒性较小。大鼠经口LD₅₀为9400mg/kg,吸入致死浓度Lc₅₀为15.33g/m³,其作业最高容许浓度为410mg/m³。然而,其嗅阈值在130~250mg/m³之间,即使在还未达到毒性浓度之前,强烈的气味就已经让人难以忍受。人体皮肤接触甲基丙烯酸甲酯后,只有极少数人会出现红疹。
-
由于生产甲基丙烯酸甲酯的原料氰化钠和中间体氰化氢均剧毒,因此必须严格确保生产设备密闭,并保持良好的通风条件。操作人员应配备必要的防护装备,并在发现中毒现象时立即移至空气新鲜处进行及时抢救。
-
稳定性:稳定。
-
禁配物:氧化剂、酸类、碱类、还原剂、过氧化物、胺类及卤素。
-
应避免接触的条件:受热、光照、紫外线以及与空气接触。
-
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:7
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
ADMET
安全信息
-
职业暴露等级:A
-
职业暴露限值:TWA: 100 ppm (410 mg/m3)
-
TSCA:Yes
-
危险等级:3
-
立即威胁生命和健康浓度:1,000 ppm
-
危险品标志:F
-
安全说明:S24,S37,S46
-
危险类别码:R43,R11,R37/38
-
WGK Germany:1
-
海关编码:3824909990
-
危险品运输编号:UN 1247
-
RTECS号:OZ5075000
-
包装等级:II
-
储存条件:储存注意事项:通常商品会添加阻聚剂。应将物品存放于阴凉、通风的库房中,远离火种和热源,并避免阳光直射。库温不宜超过37℃。包装需密封,不可与空气接触。与氧化剂、酸类、碱类、卤素等物质要分开存放,切忌混储。不宜大量储存或长期存放。照明和通风设施应采用防爆型,禁止使用易产生火花的机械设备和工具。储存区域应配备泄漏应急处理设备及合适的收容材料。
SDS
| 国标编号: | 32149 |
| CAS: | 80-62-6 |
| 中文名称: | 甲基丙烯酸甲酯 |
| 英文名称: | Methyl methacrylate;Methacrylic acid,methyl ester |
| 别 名: | 异丁烯酸甲酯;牙托水;有机玻璃单体 |
| 分子式: | C 5 H 8 O 2 ;CH 2 C(CH 3 )COOCH 3 |
| 分子量: | 100.12 |
| 熔 点: | -50℃ 沸点:101℃ |
| 密 度: | 相对密度(水=1);相对 |
| 蒸汽压: | 10℃ |
| 溶解性: | 微溶于水,溶于乙醇等 |
| 稳定性: | 稳定 |
| 外观与性状: | 无色易挥发液体,并具有强辣味 |
| 危险标记: | 7(易燃液体) |
| 用 途: | 用作有机玻璃的单体,也用于制造其它树脂、塑料、涂料、粘合剂、润滑剂 |
2.对环境的影响:
一、健康危害
侵入途径:吸入、食入。
健康危害:人对本品气味感觉阈浓度为85mg/m 3 ,刺激作用阈浓度(暴露1分钟)为285mg/m 3 。中毒表现为乏力、恶心、反复呕吐、头痛、头晕、胸闷、伴有短暂的意识消失、中性白细胞增多症。慢性中毒:神经系统受损的综合症状占主要地位,个别可发生中毒性脑病。可引起轻度皮炎和结膜炎。接触时间长可致麻醉作用。
二、毒理学资料及环境行为
毒性:为麻醉剂,麻醉浓度和致死浓度几乎相同,有弱的刺激作用。
急性毒性:LD 50 7872mg/kg(大鼠经口);LC 50 3750ppm(大鼠吸入);人吸入725ppm,最小致死浓度;人吸入62ppm×20~90分钟,粘膜刺激;人吸入12.5~25ppm×20~90分钟,头晕,恶心,意识障碍。
亚急性和慢性毒性:狗吸入46800ppm×1.5小时/日×8日,绝对致死浓度,肝、肾有损害。
致突变性:微粒体致突变:鼠伤寒沙门氏菌34mmol/L。
生殖毒性:大鼠吸入最低中毒浓度(TCL 0 ):109g/kg(孕6~15天用药),致胚胎毒性,对肌肉骨骼系统有影响。
危险特性:遇明火、高热或与氧化剂接触,有引起燃烧爆炸的危险。若遇高热,可能发生聚合反应,出现大量放热现象,引起容器破裂和爆炸事故。其蒸气比空气重,能在较低处扩散到相当远的地方,遇明火会引着回燃。
燃烧(分解)产物:一氧化碳、二氧化碳。
3.现场应急监测方法:
4.实验室监测方法:
气相色谱法《空气中有害物质的测定方法》,杭士平主编
羟胺-氯化铁比色法《空气中有害物质的测定方法》,杭士平主编
5.环境标准:
| 前苏联 | 车间空气中有害物质的最高容许浓度 | 10 mg/m 3 |
| 前苏联(1975) | 居民区大气中有害物最大允许浓度 | 0.1mg/m 3 (最大值,昼夜均值) |
| 前苏联(1975) | 水体中有害有机物的最大允许浓度 | 0.01mg/L |
| 嗅觉阈浓度 | 0.21ppm |
6.应急处理处置方法:
一、泄漏应急处理
切断火源。戴自给式呼吸器,穿一般消防防护服。在确保安全情况下堵漏。喷水雾可减少蒸发。用砂土、蛭石或其它惰性材料吸收,然后运至空旷的地方掩埋、蒸发、或焚烧。或用不燃性分散剂制成的乳液刷洗,经稀释的洗液放入废水系统。如大量泄漏,利用围堤收容,然后收集、转移、回收或无害处理后废弃。
二、防护措施
呼吸系统防护:空气中浓度较高时,建议佩戴防毒面具。
眼睛防护:戴化学安全防护眼镜。
防护服:穿防静电工作服。
手防护:必要时戴防护手套。
其它:工作现场严禁吸烟。工作后,淋浴更衣。注意个人清洁卫生。
三、急救措施
皮肤接触:脱去污染的衣着,用肥皂水及清水彻底冲洗。
眼睛接触:立即翻开上下眼睑,用流动清水冲洗15分钟。就医。
吸入:脱离现场至空气新鲜处。呼吸困难时给输氧。呼吸停止时,立即进行人工呼吸。
食入:误服者给饮足量温水,催吐,就医。
灭火方法:雾状水、泡沫、二氧化碳、干粉、砂土。
制备方法与用途
聚甲基丙烯酸酯,全称聚甲基丙烯酸甲酯(Polymethacrylates,PMMA),是一种重要的光学透明塑料。它具有高透明度、优良的耐冲击性能、轻质、经济实惠及环保无毒等优点,广泛用作玻璃替代材料。最显著的特点是其优异的光学性能,这也导致了它俗称“有机玻璃”的原因。由于它是无定型聚合物,分子排列均匀不会影响光线在内部各部分通过的速度,使得光线下行畅通无阻。
然而,尽管拥有诸多优点,PMMA也存在表面硬度低、耐磨性差以及容易受到化学溶剂侵蚀等缺点。因此,在使用之前通常需对其进行一定的表面处理以达到扬长避短的效果。
PMMA的表面改性PMMA进行表面改性的常用方法是涂覆一层耐磨加硬材料。常见的塑料耐磨加硬材料可以分为两大类:一类是以多官能度丙烯酸酯为主体的紫外光固化硬质涂层体系;另一类则是以聚硅氧烷预聚体为主的热固型有机硅硬质涂层体系。
生产方法PMMA的生产可通过多种途径进行:
- 丙酮氰醇法:首先将丙酮与氰化氢反应生成丙酮氰醇,随后与硫酸及甲醇反应制得甲基丙烯酸甲酯。
- 丙烯法:丙烯、一氧化碳和甲醇通过羰化反应合成中间体,再将其分解成目标产物。
- 异丁烯法:在钼催化剂存在下用空气对异丁烯进行两段氧化,生成甲基丙烯酸甲酯。
- 一步氧化法:以杂多酸及其盐类为催化剂,直接将异丁醛氧化生成甲基丙烯酸,再与甲醇反应制得目标产物。
- 分类:易燃液体
- 毒性分级:中毒
- 口服LD50(大鼠):7872 mg/kg;口服LD50(小鼠):3625 mg/kg
- 刺激数据:
- 眼睛刺激:兔子 150 mg
- 皮肤刺激:兔子 10,000 mg/kg
- 爆炸物危险特性:与空气混合可爆
- 可燃性危险特性:遇明火、高温或氧化剂易燃;燃烧时产生刺激烟雾
- 储运特性:应储存在通风良好且低温干燥的地方,避免与酸类及氧化剂接触
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 甲基丙烯酸 Methacrylic acid 79-41-4 C4H6O2 86.0904 甲基丙烯酸羟乙酯 2-Hydroxyethyl methacrylate 868-77-9 C6H10O3 130.144 丙酸甲酯 Methyl propionate 554-12-1 C4H8O2 88.1063 甲基丙烯酸丁酯 2-methyl-butyl acrylate 97-88-1 C8H14O2 142.198 乙二醇二甲基丙烯酸酯 EDMA 97-90-5 C10H14O4 198.219 丙烯酸乙酯 ethyl acrylate 140-88-5 C5H8O2 100.117 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 甲基丙烯酸乙酯 ethyl methacrylate 97-63-2 C6H10O2 114.144 惕各酸甲酯 methyl (E)-2-methyl-2-butenoate 6622-76-0 C6H10O2 114.144 —— methyl (E)-3-iodo-2-methylacrylate 220525-86-0 C5H7IO2 226.014 3-溴-2-甲基甲基丙烯酸酯 methyl (E)-3-bromo-2-methyl-2-propenoate 40053-01-8 C5H7BrO2 179.013 —— methyl 3-bromo-2-methyl-2-propenoate 40053-01-8 C5H7BrO2 179.013 甲基丙烯酸 Methacrylic acid 79-41-4 C4H6O2 86.0904 2-(氯甲基)丙烯酸甲酯 methyl 2-(chloromethyl)acrylate 922-15-6 C5H7ClO2 134.562 2-(溴甲基)丙烯酸甲酯 methyl 2-(bromomethyl)propenoate 4224-69-5 C5H7BrO2 179.013 甲基丙烯酸烯丙酯 allyl methacrylate 96-05-9 C7H10O2 126.155 丙基-2-甲基-2-丙烯酸酯 propyl methacrylate 2210-28-8 C7H12O2 128.171 甲基丙烯酸羟乙酯 2-Hydroxyethyl methacrylate 868-77-9 C6H10O3 130.144 2-氯乙基甲基丙烯酸盐 2-chloroethyl methacrylate 1888-94-4 C6H9ClO2 148.589 2-溴甲基丙烯酸乙酯 2-bromoethyl methacrylate 4513-56-8 C6H9BrO2 193.04 —— 2-iodoethyl 2-methyl-2-propenoate 35531-61-4 C6H9IO2 240.041 丙酸甲酯 Methyl propionate 554-12-1 C4H8O2 88.1063 甲基丙烯酸甲基烯丙酯 methallyl methacrylate 816-74-0 C8H12O2 140.182 —— methacrylic acid acetate 76392-13-7 C6H8O3 128.128 甲基丙烯酸丁酯 2-methyl-butyl acrylate 97-88-1 C8H14O2 142.198 —— 2-methyl-acrylic acid but-3-enyl ester 79964-12-8 C8H12O2 140.182 甲基丙烯酸3-羥丙酯 2-methylacrylic acid 3-hydroxypropyl ester 2761-09-3 C7H12O3 144.17 —— Methyl 3-methoxy-2-methyl-2-propenoate 82387-42-6 C6H10O3 130.144 3-甲氧基甲基丙烯酸甲酯 methyl β-methoxymethacrylate 82387-42-6 C6H10O3 130.144 反-2-甲基-2-戊烯酸甲酯 methyl (2E)-2-methyl-2-pentenoate 1567-14-2 C7H12O2 128.171 甲基丙烯酸异丁酯 Isobutyl methacrylate 97-86-9 C8H14O2 142.198 甲基丙烯酸甲氧基乙酯 2-methoxyethyl methacrylate 6976-93-8 C7H12O3 144.17 —— 3-chloropropyl methacrylate 44903-02-8 C7H11ClO2 162.616 —— Methyl 3-cyano-2-methylprop-2-enoate 66396-68-7 C6H7NO2 125.127 甲基丙烯酸-2-乙氧基乙酯 2-ethoxyethyl methacrylate 2370-63-0 C8H14O3 158.197 3-(2-甲基丙-2-烯酰氧基)丙基2-甲基丙-2-烯酸酯 triethylene glycol dimethacrylate 1188-09-6 C11H16O4 212.246 —— Methyl 2-methyl-3-(methylamino)prop-2-enoate 33523-89-6 C6H11NO2 129.159 甲基丙烯酸4-羟基丁酯 4-hydroxybutyl methacrylate 997-46-6 C8H14O3 158.197 乙二醇二甲基丙烯酸酯 EDMA 97-90-5 C10H14O4 198.219 甲基丙烯酸戊酯 butyl-methyl methacrylate 2849-98-1 C9H16O2 156.225 1,4-丁二醇二甲基丙烯酸酯 1,4-butanediol dimethacrylate 2082-81-7 C12H18O4 226.273 —— pent-4-en-1-ol methacrylate 84461-12-1 C9H14O2 154.209 —— methylpropenoic acid 4-(propenoyloxy)butyl ester 210967-81-0 C11H16O4 212.246 三乙二醇二甲基丙烯酸酯 triethyleneglycol dimethacrylate 109-16-0 C14H22O6 286.325 二乙二醇二甲基丙烯酸酯 diethylene glycol dimethacrylate 2358-84-1 C12H18O5 242.272 3-甲基丁烯-2-基甲基丙烯酸酯 prenyl methacrylate 85269-36-9 C9H14O2 154.209 四乙二醇二甲基丙烯酸酯 tetraethylene glycol dimethacrylate 109-17-1 C16H26O7 330.378 聚乙二醇单马来酸酯 2-(2-hydroxyethoxy)ethyl methacrylate 2351-43-1 C8H14O4 174.197 3,6,9,12-四氧杂十三烷-1-基甲基丙烯酸酯 methoxytetraethyleneglycol methacrylate 57454-26-9 C13H24O6 276.33 —— β-chloromethacrylic acid 6625-00-9 C4H5ClO2 120.535 —— methacrylic acid-(5-hydroxy-pentyl ester) 61016-96-4 C9H16O3 172.224 1,5-二甲基丙烯酸戊二醇酯 1,5-pentanediol di(meth)acrylate 13675-34-8 C13H20O4 240.299 甲基丙烯酸异戊酯 iso-amyl methacrylate 7336-27-8 C9H16O2 156.225 - 1
- 2
- 3
- 4
- 5
反应信息
-
作为反应物:参考文献:名称:β-cyclodextrin and hydridopentacyanocobaltate catalyzed selective hydrogenation of α,β,-unsaturated acids and their derivatives摘要:DOI:10.1016/s0040-4039(00)88883-8
-
作为产物:参考文献:名称:METHOD FOR HYDROLYSING ACETONE CYANOHYDRIN摘要:本发明涉及一种通过硫酸对丙酮氰醇(ACH)进行水解的过程,用于制备甲基丙烯酸(MAA)或甲基丙烯酸甲酯(MMA)的ACH磺酸法过程。公开号:US20150045577A1
-
作为试剂:描述:2,2,6,6-四甲基哌啶氧化物 、 4-甲苯硫酚 在 甲基丙烯酸甲酯 作用下, 以 1,2-二氯乙烷 、 乙腈 为溶剂, 反应 1.0h, 生成 2,2,6,6-tetramethyl-1-((p-tolylthio)oxy)piperidine参考文献:名称:Auto-oxidative hydroxysulfenylation of alkenes摘要:一锅法介导的电子不足和电子富集烯烃与苯硫醇的羟基硫醚化反应被探索。DOI:10.1039/c6cc01937d
文献信息
-
[EN] BICYCLIC ARYL SPHINGOSINE 1-PHOSPHATE ANALOGS<br/>[FR] ANALOGUES D’ARYLSPHINGOSINE-1-PHOSPHATE BICYCLIQUES申请人:BIOGEN IDEC INC公开号:WO2011017561A1公开(公告)日:2011-02-10Compounds that have agonist activity at one or more of the SlP receptors are provided. The compounds are sphingosine analogs that, after phosphorylation, can behave as agonists at SlP receptors.
-
[EN] COMPOUNDS AND THEIR USE AS BACE INHIBITORS<br/>[FR] COMPOSÉS ET LEUR UTILISATION EN TANT QU'INHIBITEURS DE BACE申请人:ASTRAZENECA AB公开号:WO2016055858A1公开(公告)日:2016-04-14The present application relates to compounds of formula (I), (la), or (lb) and their pharmaceutical compositions/preparations. This application further relates to methods of treating or preventing Αβ-related pathologies such as Down's syndrome, β- amyloid angiopathy such as but not limited to cerebral amyloid angiopathy or hereditary cerebral hemorrhage, disorders associated with cognitive impairment such as but not limited to MCI ("mild cognitive impairment"), Alzheimer's disease, memory loss, attention deficit symptoms associated with Alzheimer's disease, neurodegeneration associated with diseases such as Alzheimer's disease or dementia, including dementia of mixed vascular and degenerative origin, pre-senile dementia, senile dementia and dementia associated with Parkinson's disease.本申请涉及式(I)、(Ia)或(Ib)的化合物及其药物组合物/制剂。本申请进一步涉及治疗或预防与Αβ相关的病理学,如唐氏综合症,β-淀粉样蛋白血管病,如但不限于脑淀粉样蛋白血管病或遗传性脑出血,与认知损害相关的疾病,如但不限于MCI(“轻度认知损害”),阿尔茨海默病,记忆丧失,与阿尔茨海默病相关的注意力缺陷症状,与疾病如阿尔茨海默病或痴呆症相关的神经退行性疾病,包括混合性血管性和退行性起源的痴呆,早老性痴呆,老年性痴呆和与帕金森病相关的痴呆的方法。
-
Total Synthesis of Hybocarpone and Analogues Thereof. A Facile Dimerization of Naphthazarins to Pentacyclic Systems作者:K. C. Nicolaou、David L. F. GrayDOI:10.1021/ja030497n日期:2004.1.1The total synthesis of the lichen-derived antitumor agent hybocarpone (1) and related compounds is described. The successful route to hybocarpone features a novel radical-based dimerization/hydration cascade which generates the bridging hindered carbon-carbon bond of the molecule in a stereocontrolled manner, setting the relative configurations of the four contiguous stereocenters in a single step
-
Ultra‐High‐Molecular‐Weight Polymers Produced by the Immortal Phosphine‐Based Catalyst System作者:Yun Bai、Jianghua He、Yuetao ZhangDOI:10.1002/anie.201811946日期:2018.12.21promote the frustrated Lewis pair (FLP)‐catalyzed living polymerization of methyl methacrylate (MMA), achieving ultrahigh molecular weight (UHMW) poly(methyl methacrylate) (PMMA) with Mn up to 1927 kg mol−1 and narrow molecular weight distribution (MWD) at room temperature (RT). This FLP catalyst system exhibits exceptionally long lifetime polymerization performance even in the absence of free MMA, which强大的有机磷超碱N-(二苯基膦基)-1,3-二异丙基-4,5-二甲基-1,3-二氢-2H-咪唑-2-亚胺(IAP3)与位阻但适度为酸性的路易斯酸结合使用(LA),(4-Me- 2,6 - t Bu 2 -C 6 H 2 O)Al i Bu 2((BHT)Al i Bu 2),以协同促进沮丧的Lewis对(FLP)催化的生活聚合甲基丙烯酸甲酯(MMA),实现M n高达1927 kg mol -1的超高分子量(UHMW)聚甲基丙烯酸甲酯(PMMA)和在室温(RT)下较窄的分子量分布(MWD)。该FLP催化剂体系即使在不存在游离MMA的情况下,也表现出异常长的聚合寿命,这可以在将所得体系在室温下放置24小时后重新引发所需的活性聚合。
-
Bifunctional Iminophosphorane Catalyzed Enantioselective Sulfa-Michael Addition to Unactivated α-Substituted Acrylate Esters作者:Alistair J. M. Farley、Christopher Sandford、Darren J. DixonDOI:10.1021/jacs.5b10226日期:2015.12.30The highly enantioselective sulfa-Michael addition of alkyl thiols to unactivated α-substituted acrylate esters catalyzed by a bifunctional iminophosphorane organocatalyst under mild conditions is described. The strong Brønsted basicity of the iminophosphorane moiety of the catalyst provides the necessary activation of the alkyl thiol pro-nucleophile, while the two tert-leucine residues flanking a
表征谱图
-
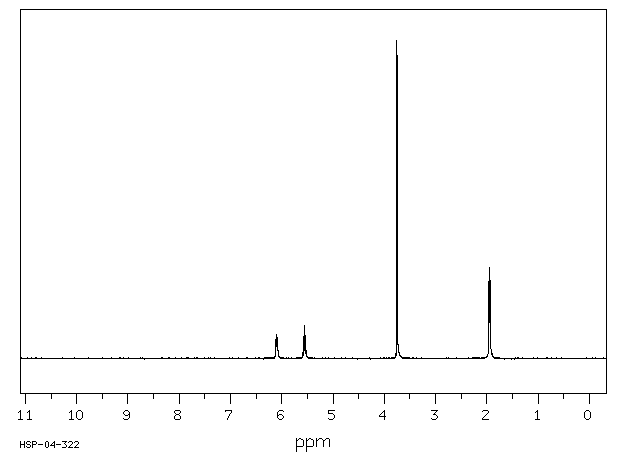
氢谱1HNMR
-
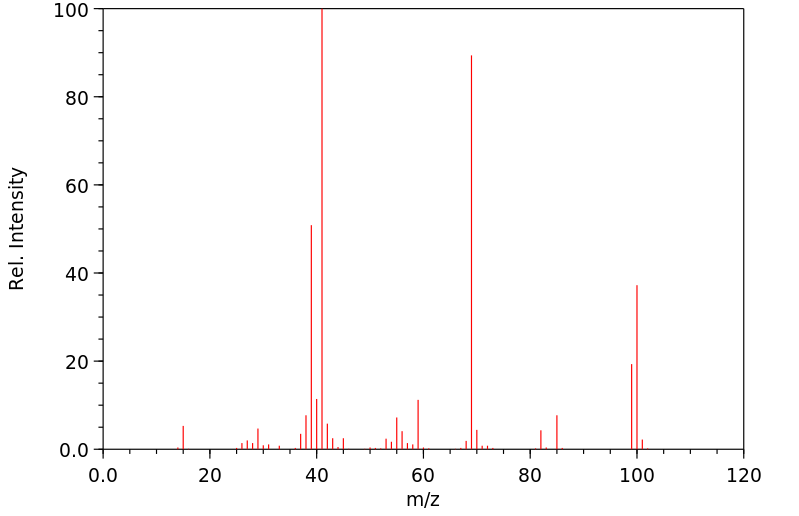
质谱MS
-
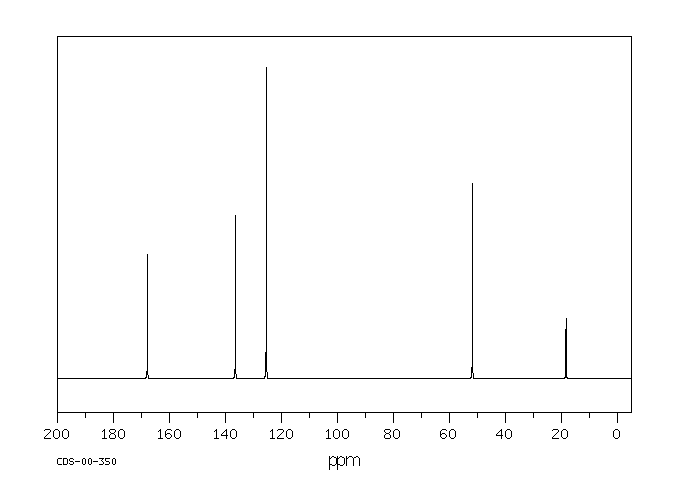
碳谱13CNMR
-
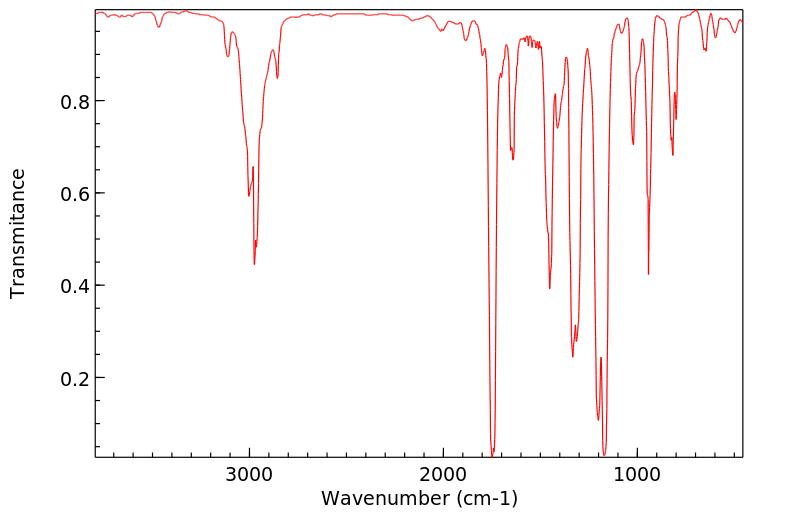
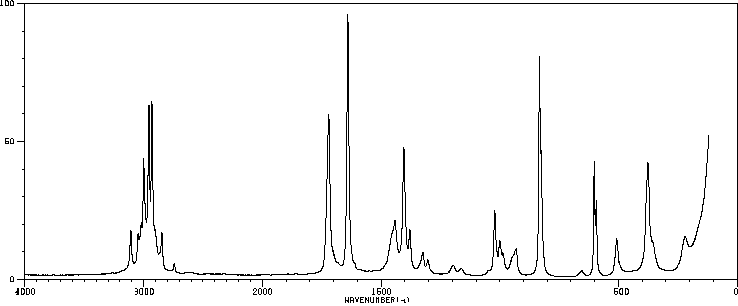
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息