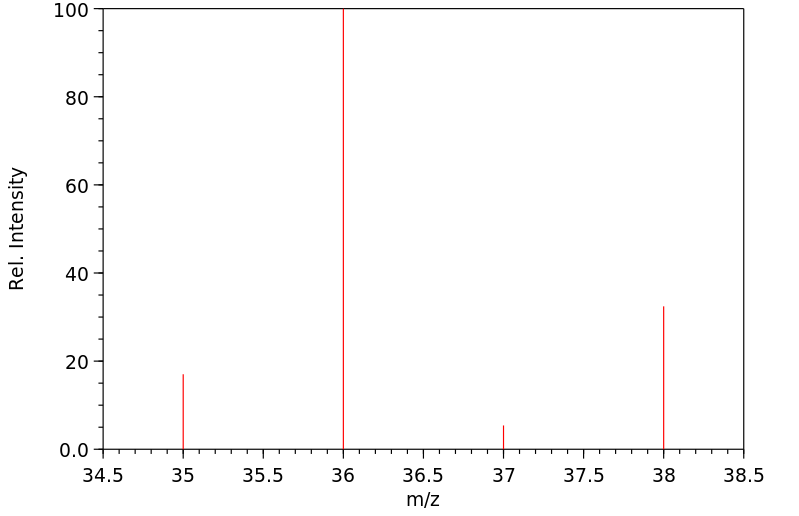
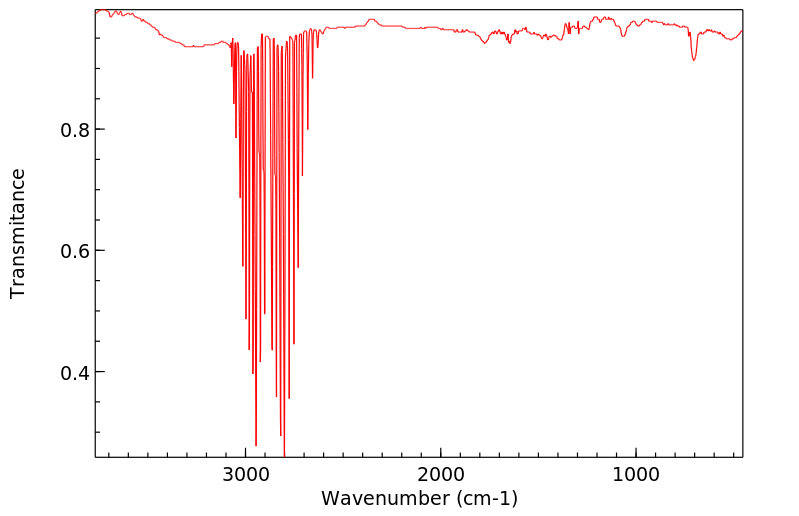
毒理性
氯化氢是一种无色气体,具有刺激性、刺激性的气味。它被用作结核病杀菌剂、消毒剂(杀菌剂/杀真菌剂/净化剂,有限的,通用的或广谱的,医院或医疗的)、消毒剂、杀病毒剂、杀真菌剂/抑真菌剂,以及杀微生物剂/抑微生物剂(形成粘液的细菌)。它还用于制造药物氯化物、从乙炔制取氯乙烯、从烯烃制取烷基氯化物和从氧化砷制取砷氯化物。在橡胶氯化中。在涉及异构化、聚合和烷基化的有机反应中。在制造氯气的地方经济实惠。氢氯酸已被确认为在液压致裂中作为pH调节剂使用。
人类暴露和毒性:氯化氢会迅速解离,其效果被认为是pH变化(局部沉积H+)的结果,而不是氯化氢/盐酸的影响。氯化氢对皮肤有腐蚀性,暴露于眼睛可能会造成严重的影响。没有报告皮肤过敏。氯化氢对粘膜的刺激如此严重,以至于工人们在检测到它的气味后很快就离开了工作场所。在人类中,没有观察到氯化氢暴露与肿瘤发病率之间的关联。在一项研究中,8名哮喘志愿者中有1人在3分钟内吸入pH为2的未缓冲盐酸气溶胶,气道阻力增加了50%。短期暴露据报道会在呼吸道引起暂时性阻塞,这种阻塞会随着反复暴露而减少,这表明了适应性。适应的工人可以在15毫克/立方米(10 ppm)的氯化氢水平下不受干扰地工作。暴露于盐酸可能会在皮肤和粘膜上产生烧伤,其严重程度与溶液的浓度有关。随后,可能会发生溃疡,接着是瘢痕疙瘩和收缩性疤痕。接触眼睛可能会导致视力下降或失明。经常接触盐酸的水溶液可能会导致皮炎。在暴露于盐酸后,牙齿腐烂、牙齿结构变化、变黄、软化破裂以及相关的消化系统疾病很常见。
动物研究:对于重复剂量毒性,在90天吸入研究中,10 ppm及以上的组观察到局部刺激作用。对于遗传毒性, Ames试验显示阴性结果。在使用仓鼠卵巢细胞的染色体畸变试验中,获得了阳性结果,这被认为是由于低pH造成的假象。对于致癌性,在128周吸入研究中,雄性大鼠在10 ppm氯化氢气体下没有观察到前肿瘤或肿瘤性鼻病变。在其他通过吸入、口服或皮肤给药进行的动物研究中也没有观察到与治疗相关的致癌性。氯化氢不期望具有发育毒性。此外,在一个良好的90天吸入研究中,直到50 ppm也没有观察到对性腺的影响。
生态毒性研究:盐酸对环境的危害是由质子(pH效应)引起的。因此,盐酸对生物体的影响取决于水生生态系统的缓冲能力。急性鱼类毒性试验的LC50值的变化也可以在很大程度上由试验介质的缓冲能力的差异来解释。例如,急性鱼毒性试验的LC50值从4.92到282毫克/升不等。
IDENTIFICATION AND USE: Hydrogen chloride is a colorless gas with pungent, irritating odor. it is used as tuberculocide, disinfectant (bactericide/germicide/purifier, limited, general or broad-spectrum, hospital or medical), sanitizer, virucide, fungicide/fungistat, and microbicide/microbiostat (slime-forming bacteria). It is also used in the manufacture of pharmaceutical hydrochlorides, vinyl chloride from acetylene, alkyl chlorides from olefins, and arsenious chlorides from arsenious oxide. In the chlorination of rubber. In organic reactions involving isomerization, polymerization, and alkylation. For making chlorine where economical. Hydrochloric acid has been identified as being used in hydraulic fracturing as a pH adjuster. HUMAN EXPOSURE AND TOXICITY: Hydrogen chloride will rapidly dissociate and its effects are thought to be a result of pH change (local deposition of H+) rather than effects of hydrogen chloride/hydrochloric acid. Hydrogen chloride is corrosive to the skin and severe effects can be expected from exposure to the eyes. No skin sensitization has been reported. The irritation of hydrogen chloride to mucous is so severe that workers evacuate from the work place shortly after detecting its odor. In humans, no association between hydrogen chloride exposure and tumor incidence was observed. In one of eight asthmatic volunteers exposed to an aerosol of unbuffered hydrochloric acid at pH 2 for 3 min during tidal breathing, airway resistance was increased by 50%. Short term exposures have been reported to induce transitory obstruction in the respiratory tract, which diminishes with repeated exposure, suggesting adaption. Acclimatized workers can work undisturbed with a hydrogen chloride level of 15 mg/cu m (10 ppm). Exposure to hydrochloric acid can produce burns on the skin and mucous membranes, the severity of which is related to the concentration of the solution. Subsequently, ulceration may occur, followed by keloid and retractile scarring. Contact with the eyes may produce reduced vision or blindness. Frequent contact with aqueous solutions of hydrochloric acid may lead to dermatitis. Dental decay, with changes in tooth structure, yellowing, softening and breaking of teeth, and related digestive diseases are frequent after exposures to hydrochloric acid. ANIMAL STUDIES: For repeated dose toxicity, local irritation effects were observed in the groups of 10 ppm and above in a 90-day inhalation study. For genetic toxicity, a negative result has been shown in the Ames test. A positive result, which is considered to be an artifact due to the low pH, has been obtained in a chromosome aberration test using Hamster ovary cells. For carcinogenicity, no pre-neoplastic or neoplastic nasal lesions were observed in a 128-week inhalation study with male rats at 10 ppm hydrogen chloride gas. No evidence of treatment related carcinogenicity was observed either in other animal studies performed by inhalation, oral or dermal administration. Hydrogen chloride is not expected to have developmental toxicity. In addition, no effects on the gonads were observed in a good 90- day inhalation study up to 50 ppm. ECOTOXICITY STUDIES: The hazard of hydrochloric acid for the environment is caused by the proton (pH effect). For this reason the effect of hydrochloric acid on the organisms depends on the buffer capacity of the aquatic ecosystem. Also the variation in acute toxicity for aquatic organisms can be explained for a significant extent by the variation in buffer capacity of the test medium. For example, LC50 values of acute fish toxicity tests varied from 4.92 to 282 mg/L
来源:Hazardous Substances Data Bank (HSDB)