红藻胶 | 9000-21-9
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):6.1
-
重原子数:36
-
可旋转键数:9
-
环数:4.0
-
sp3杂化的碳原子比例:0.16
-
拓扑面积:64.6
-
氢给体数:1
-
氢受体数:5
制备方法与用途
红藻胶为白色至浅黄棕色粉末,无臭且味微咸。它易溶于75~77℃的水和牛奶中,形成坚实、光滑、有弹性的凝胶。1.5%的水分散液在37℃时产生粘度,在43℃达到最大值,超过此温度则粘度降低。红藻胶的质量即使经过数小时也不易下降,其凝胶与琼脂一样坚硬,并且比卡拉胶更为坚固。钾离子的存在会增加凝胶强度,而凝胶强度也随pH的变化而变化,pH为8时达到最高值,在低pH区域加热则降低。红藻胶不溶于乙醇。
用途 使用范围根据国家标准,建议红藻胶的添加量为千分之一到千分之三。
毒性ADI未作特殊规定(FAO/WHO,2001)。可安全用于食品(FDA,§172.655,2000),大鼠经口LD₅₀为5000mg/kg。
使用限量FAO/WH0 (1984, g/kg):青刀豆和黄刀豆、甜玉米、蘑菇、芦笋、青豌豆等罐头产品中含奶油或其他油脂者为10;酪农干酪按稀奶油计为5;酸黄瓜为500mg/kg;肉汤、羹为5000mg/kg;胡萝卜罐头为10;发酵后经热处理的增香酸奶及其制品为5000mg/kg。
红藻胶还可用于牛奶布丁、牛奶冻、果酱、果冻和橘皮果冻、面包用果冻、冰淇淋、冰糕、乳蛋羹、掼奶油、洋火腿罐头、肉类及鱼类的贮藏、疗效食品等。
化学性质红藻胶为白色至浅黄棕色粉末,无臭且味微咸。它易溶于75~77℃的水和牛奶中,形成坚实、光滑、有弹性的凝胶。1.5%的水分散液在37℃时产生粘度,在43℃达到最大值,超过此温度则粘度降低。红藻胶的质量即使经过数小时也不易下降,其凝胶与琼脂一样坚硬,并且比卡拉胶更为坚固。钾离子的存在会增加凝胶强度,而凝胶强度也随pH的变化而变化,pH为8时达到最高值,在低pH区域加热则降低。红藻胶不溶于乙醇。
生产方法红藻胶由红藻类(Rhodophyceae)帚叉藻族(F“Mellar’in)的FAstigzata海藻在日光下晒干,然后用碱处理2~3周。经过中和、水洗、煮沸、抽提、离心分离后,在澄清液中添加1%的氯化钾溶液形成凝胶。将此凝胶于-16°C冷冻脱水,并在隧道式干燥机中于70℃下干燥,得率为原藻的2.5%~3.5%。红藻胶主要产自丹麦、挪威等国家海域。
反应信息
-
作为反应物:参考文献:名称:6-Phenyl-1,4-dihydropyridine Derivatives as Potent and Selective A3 Adenosine Receptor Antagonists摘要:An approach to designing dihydropyridines that bind to adenosine receptors without binding to L-type calcium channels has been described. 1,4-Dihydropyridine derivatives substituted with beta-styryl or phenylethynyl groups at the 4-position and aryl groups at the 6-position were synthesized and found to be selective for human A(3) receptors. Combinations of methyl, ethyl, and benzyl esters were included at the 3- and 5-positions. Affinity was determined in radioligand binding assays at rat brain A(1) and A(2A) receptors using [H-3]-(R)-PIA [[H-3]-(R)-N-6-(phenylisopropyl)adenosine] and [H-3]CGS 21680 [[H-3]-2-[[4-(2-carboxymethyl)phenyl]ethylamino]-5'-(N-ethyl carbamoyl)adenosine], respectively. Affinity was determined at cloned human and rat A(3) receptors using [I-125]AB-MECA [N-6-(4-amino-3-iodobenzyl)-5'-(N-methycarbonyl)adenosine]. Structure-activity analysis indicated that substitution of the phenyl ring of the beta-styryl group but not of the 6-phenyl substituent was tolerated in A(3) receptor selective agents. Replacement of the 6-phenyl ring with a 3-thienyl or 3-furyl group reduced the affinity at A(3) receptors by 4- and g-fold, respectively. A 5-benzyl ester 4-trans-beta-styryl derivative, 26, with a K-i value of 58.3 nM at A(3) receptors, was >1700-fold selective vs either A(1) receptors or A(2A) receptors. Shifting the benzyl ester to the 3-position lowered the affinity at A(3) receptors 3-fold. A 5-benzyl, 3-ethyl ester 4-phenylethynyl derivative, 28, displayed a K-i value of 31.4 nM at A(3) receptors and 1300-fold selectivity vs A(1) receptors. The isomeric 3-benzyl, 5-ethyl diester was >600-fold selective for A(3) receptors. Oxidation of 28 to the corresponding pyridine derivative reduced affinity at A(3) receptors by 88-fold and slightly increased affinity at A(1) receptors.DOI:10.1021/jm960457c
-
作为产物:描述:参考文献:名称:4-(苯基乙炔基)-6-苯基-1,4-二氢吡啶作为高选择性 A3 腺苷受体拮抗剂的结构-活性关系。摘要:4-(苯乙炔基)-6-苯基-1,4-二氢吡啶衍生物是人 A3 腺苷受体的选择性拮抗剂,与 [125I]AB-MECA (N6-(4-amino-3-碘苄基)-5'-(N-甲基氨基甲酰基)腺苷)在亚微摩尔范围内。在本研究中,综合探讨了二氢吡啶环不同位置(3-和5-酰基取代基、4-芳基取代基和1-甲基)的构效关系。利用1-乙氧基甲基和5-[2-(三甲基甲硅烷基)乙基]酯基团的联合保护,在5位形成游离羧酸,允许各种取代。确定新类似物对克隆人 A3 腺苷受体的选择性与大鼠脑 A1 和 A2A 受体上放射性配体的结合。腺苷受体的结构活性分析表明,4 位的吡啶基、呋喃基、苯并呋喃基和噻吩基最多只能对 A3 腺苷受体产生中等选择性。与4-苯乙烯基取代的二氢吡啶不同,4-苯乙炔基的环取代(例如4-硝基)没有提供增强的选择性。在二氢吡啶环的 3 位,酯对 A3 受体的选择性比密切相关的硫酯、酰胺和酮衍生物高得多。环状DOI:10.1021/jm970091j
文献信息
-
5-pyridyl-1, 3-azole compounds, process for producing the same and use there of申请人:Ohkawa Shigenori公开号:US20090048307A1公开(公告)日:2009-02-19An optionally N-oxidized compound represented by the formula: wherein R 1 represents hydrogen, hydrocarbon, heterocycle, amino, acyl, R 2 represents an aromatic group, R 3 represents hydrogen, pyridyl, aromatic hydrocarbon, X represents oxygen, optionally oxidized sulfur, Y represents a bond, an oxygen, optionally oxidized sulfur, a group represented by the formula NR 4 (R 4 represents hydrogen, hydrocarbon or acyl) and Z represents a bond or a divalent acyclic hydrocarbon, or a salt thereof has an excellent adenosine A 3 receptor antagonistic activity and is used as an agent for preventing or treating diseases related to an adenosine A 3 receptor. Furthermore, the compound (I) or a salt thereof has p38 MAP kinase inhibitory activity and TNF-α inhibitory activity and is used as an agent for preventing or treating diseases related to p38 MAP kinase and diseases related to TNF-α.
-
Modulation of the P2Y2 receptor pathway申请人:P2-Science APS公开号:EP2036567A2公开(公告)日:2009-03-18The present invention relates to the field of regulating the activity of the purinergic receptors for the modulation of the vascular tone, particularly for the purpose of treatment of haemodynamic conditions by overriding of vasoconstriction activity, such as increases in sympathic vasoconstriction. Modulators, such as UTP analogues as described herein are preferably specific for P2Y2. Compounds capable of stimulating the P2Y2 receptor are suitable for the treatment or prevention wherein inhibition of vasoconstriction activity is desirable such as hypertension and hypertension relates disorders or diseases, whereas compound capable of counteracting the activity of the P2Y2 receptor are suitable for the treatment of or prevention wherein inhibition of vasodilatation is desirable.本发明涉及调节嘌呤能受体活性以调节血管张力的领域,特别是通过抑制血管收缩活性(如交感血管收缩增加)来治疗血流动力学疾病。调节剂,如本文所述的UTP 类似物,最好对 P2Y2 具有特异性。能够刺激 P2Y2 受体的化合物适用于治疗或预防需要抑制血管收缩活性的疾病,如高血压和高血压相关失调或疾病,而能够抵消 P2Y2 受体活性的化合物适用于治疗或预防需要抑制血管舒张的疾病。
-
Methods for reducing intraocular pressure申请人:——公开号:US20030153626A1公开(公告)日:2003-08-14A method for decreasing intraocular pressure by administering an A 3 subtype adenosine receptor antagonist, a calmodulin antagonist or an antiestrogen such as tamoxifen. These agents, by inhibiting influx or promoting efflux of aqueous humor, can be used to treat glaucoma.
-
Purines are self-renewal signals for neural stem cells, and purine receptor antagonists promote neuronal and glial differentiation therefrom申请人:Goldman A. Steven公开号:US20050181503A1公开(公告)日:2005-08-18The present invention relates to a method of inhibiting differentiation of a population of neural stem cells by contacting a purinergic receptor agonist and a population of neural stem cells under conditions effective to inhibit differentiation of the population of neural stem cells. Another aspect of the present invention relates to a method of producing neurons and/or glial cells from a population of neural stem cells by culturing a population of neural stem cells with a purinergic receptor antagonist under conditions effective to cause the neural stem cells to differentiate into neurons and/or glial cells. The purinergic receptor agonist can also be used in a method of inducing proliferation and self-renewal of neural stem cells in a subject and a method of treating a neurological disease or neurodegenerative condition in a subject. The purinergic receptor antagonist can also be used in treating a neoplastic disease of the brain or spinal cord in a subject.
-
5-PYRIDYL-1,3-AZOLE COMPOUNDS, PROCESS FOR PRODUCING THE SAME AND USE THEREOF申请人:Takeda Pharmaceutical Company Limited公开号:EP1180518B1公开(公告)日:2007-01-17
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
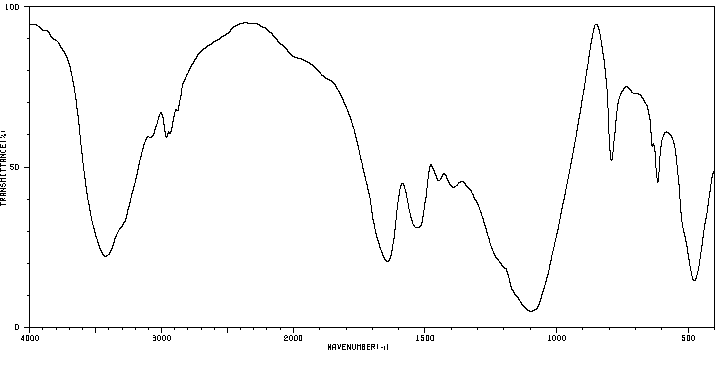
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息