胡椒酮 | 89-81-6
中文名称
胡椒酮
中文别名
3甲基-6-(1-甲基乙基)-2-环己烯-1-酮;3-甲基-6-(1-甲基乙基)-2-环己烯-1-酮
英文名称
piperitone
英文别名
piperiton;p-menth-1-en-3-one;3-methyl-6-(1-methylethyl)-2-cyclohexen-1-one;dl-Piperiton;(±)-piperitone;(+/-)-6-Isopropyl-3-methylcyclohex-2-en-1-one;6-isopropyl-3-methyl-2-cyclohexen-1-one;3-Methyl-6-isopropyl-cyclohex-2-enon;6-isopropyl-3-methylcyclohex-2-enone;3-carvomenthenone;3-methyl-6-propan-2-ylcyclohex-2-en-1-one
CAS
89-81-6
化学式
C10H16O
mdl
MFCD00045532
分子量
152.236
InChiKey
YSTPAHQEHQSRJD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-29 °C
-
沸点:233°C
-
密度:0,93 g/cm3
-
溶解度:氯仿(微溶)、乙酸乙酯(微溶)
-
LogP:2.4
-
物理描述:Solid
-
折光率:1.483-1.487
-
保留指数:1234;1246;1246;1228;1243;1232;1250;1228;1219;1226;1226;1223;1234;1223;1218;1218;1231;1233;1233;1232;1232;1235;1221;1232;1235;1236;1236;1228;1223;1255;1222;1233;1233;1237;1230;1233;1256;1231;1232;1242;1233.3;1236;1250;1221;1238;1231;1231;1229;1224;1224;1235;1237;1226;1229;1236;1229;1226;1236;1232;1236;1237;1236;1234;1233;1227;1249;1235;1221;1221;1248;1228.2;1237.4;1222;1222;1280;1224;1232;1228;1235;1236;1235;1241.2;1233;1230;1223;1235;1248;1237;1243;1245;1247;1235;1236;1223;1233;1247;1235;1225;1230;1230;1231
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.7
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
安全说明:S23
-
海关编码:2914299000
-
WGK Germany:1
-
危险性防范说明:P280
-
危险性描述:H315
-
储存条件:存于室温、干燥且密封的环境中。
SDS
Section I.Chemical Product and Company Identification
Chemical Name Piperitone
Portland OR
Synonym 2-Cyclohexen-1-one, 3-methyl-6-(1-methylethyl)-
(CA INDEX NAME); 1-p-Menthen-3-one;
6-Isopropyl-3-methyl-2-cyclohexen-1-one
Chemical Formula C10H16O
89-81-6
CAS Number
Section II. Composition and Information on Ingredients
Chemical Name CAS Number Percent (%) TLV/PEL Toxicology Data
Piperitone 89-81-6 Min. 94.0 (GC) Not available. Rat LD50 (oral) 2450 mg/kg
Mouse LD50 (subcutaneous) 1420
mg/kg
Section III. Hazards Identification
Acute Health Effects Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the
eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening, or,
occasionally, blistering.
Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound.
Chronic Health Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Section IV. First Aid Measures
Eye Contact Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15
minutes. Get medical attention.
Skin Contact In case of contact, immediately flush skin with plenty of water. Remove contaminated clothing and shoes. Wash clothing
before reuse. Thoroughly clean shoes before reuse. Get medical attention.
If the victim is not breathing, perform mouth-to-mouth resuscitation. Loosen tight clothing such as a collar, tie, belt or
Inhalation
waistband. If breathing is difficult, oxygen can be administered. Seek medical attention if respiration problems do not
improve.
INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Ingestion
Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth
resuscitation. Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic
material was ingested; the absence of such signs, however, is not conclusive.
Section V. Fire and Explosion Data
Not available.
Combustible. Auto-Ignition
Flammability
Flash Points Flammable Limits Not available.
Not available.
These products are toxic carbon oxides (CO, CO2).
Combustion Products
Fire Hazards Not available.
Risks of explosion of the product in presence of mechanical impact: Not available.
Explosion Hazards
Risks of explosion of the product in presence of static discharge: Not available.
Fire Fighting Media
SMALL FIRE: Use DRY chemical powder.
LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
and Instructions
Consult with local fire authorities before attempting large scale fire-fighting operations.
Continued on Next Page
Piperitone
Section VI. Accidental Release Measures
Spill Cleanup Combustible material. Irritating material.
Keep away from heat. Mechanical exhaust required. Stop leak if without risk. Finish cleaning the spill by rinsing any
Instructions
contaminated surfaces with copious amounts of water. Consult federal, state, and/or local authorities for assistance on
disposal.
Section VII. Handling and Storage
COMBUSTIBLE. IRRITANT. Keep away from heat. Mechanical exhaust required. Avoid excessive heat and light. Do not
Handling and Storage
breathe gas/fumes/ vapor/spray.
Information
Always store away from incompatible compounds such as oxidizing agents.
Section VIII. Exposure Controls/Personal Protection
Provide exhaust ventilation or other engineering controls to keep the airborne concentrations of vapors below their respective
Engineering Controls
threshold limit value. Ensure that eyewash station and safety shower is proximal to the work-station location.
Splash goggles. Lab coat. Vapor respirator. Boots. Gloves. Suggested protective clothing might not be sufficient; consult a
Personal Protection
specialist BEFORE handling this product. Be sure to use a MSHA/NIOSH approved respirator or equivalent.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Liquid. (Clear, light yellow ~ yellow.) Solubility
Physical state @ 20°C Insoluble in water.
0.94 (water=1)
Specific Gravity
Molecular Weight 152.23 Partition Coefficient
LOG Pow: 2.5
Boiling Point 235°C (455°F) Vapor Pressure Not available.
-29°C (-20.2°F) Not available.
Melting Point Vapor Density
Refractive Index 1.48 Volatility Not available.
Not available. Not available.
Critical Temperature Odor
Viscosity Not available. Taste Not available.
Section X. Stability and Reactivity Data
This material is stable if stored under proper conditions. (See Section VII for instructions)
Stability
Conditions of Instability Avoid excessive heat and light.
Incompatibilities
Reactive with oxidizing agents.
Section XI. Toxicological Information
OT0257000
RTECS Number
Eye Contact. Ingestion. Inhalation.
Routes of Exposure
Rat LD50 (oral) 2450 mg/kg
Toxicity Data
Mouse LD50 (subcutaneous) 1420 mg/kg
CARCINOGENIC EFFECTS : Not available.
Chronic Toxic Effects
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the eye
Acute Toxic Effects
is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening, or,
occasionally, blistering.
Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound.
Section XII. Ecological Information
Ecotoxicity Not available.
Not available.
Environmental Fate
Continued on Next Page
Piperitone
Section XIII. Disposal Considerations
Waste Disposal Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material with a
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state and local regulations when disposing of the substance.
Section XIV. Transport Information
Not a DOT controlled material (United States).
DOT Classification
PIN Number Not applicable.
Proper Shipping Name Not applicable.
Packing Group (PG) Not applicable.
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This compound is ON the EPA Toxic Substances Control Act (TSCA) inventory list.
(EPA)
WHMIS Classification CLASS B-3: Combustible liquid with a flash point between 37.8°C (100°F) and 93.3°C (200°F).
On DSL.
(Canada)
EINECS Number (EEC) 201-942-7
EEC Risk Statements R36/37/38- Irritating to eyes, respiratory system and skin.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
化学性质
单环单萜类化合物,具有一对对映体,均天然存在。右旋体有樟脑气味,为油状液体,沸点在232-235℃之间,相对密度0.9344,折光率1.4848,旋光度[α]20D +49.13°。左旋体的沸点则为109.5-110.5℃(1.99kPa),相对密度0.9324,折光率1.4832。这两种光学异构体常分别存在于多种精油中。
用途
用于薄荷脑和麝香草酚的合成,并应用于化妆品、牙膏香精等领域。
生产方法
右旋体在臭草油中的含量可达80%,而左旋体则主要存在于多种桉树油中,如阔叶桉(Eucalyptus dives)油。通过减压分馏和重亚硫酸钠加成法可以从这些油中分离并提纯这两种单环单萜类化合物。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— p-Menthen-1(6)-on-3 64853-97-0 C10H16O 152.236 (+)-对-薄荷-1-烯 (+)-p-menth-1-ene 1195-31-9 C10H18 138.253 —— p-menth-1-ene 27966-26-3 C10H18 138.253 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5-异丙基-2-甲基-2-环己烯-1-酮 carvotanacetone 43205-82-9 C10H16O 152.236 2-羟基-6-(异丙基)-3-甲基环己-2-烯-1-酮 diosphenol 490-03-9 C10H16O2 168.236 —— trans-5-hydroxypiperitone —— C10H16O2 168.236 —— 4-hydroxypiperitone 1197-72-4 C10H16O2 168.236 3-甲基-2-环己烯-1-酮 3-methylcyclohexen-2-one 1193-18-6 C7H10O 110.156
反应信息
-
作为反应物:参考文献:名称:Treibs, Chemische Berichte, 1928, vol. 61, p. 685摘要:DOI:
-
作为产物:描述:参考文献:名称:环结构中的α-烯酮烷基和α,β-不饱和酰基自由基中间体摘要:用Bu 3 SnH-AIBN处理E - α,β-不饱和硒烯基酯1和3a分别产生相应的环己烯酮2,并通过假定的α-烯酮烷基自由基中间体产生相应的环己烯酮2。以类似的方式,环丙基酯9产生12和13的混合物,并且2,7-二烯硒烯基酯15经历新的双环化,以76%的产率产生二喹烷17。DOI:10.1016/0040-4039(95)02147-7
文献信息
-
Linderapyrone: A Wnt signal inhibitor isolated from Lindera umbellata作者:Takahiro Matsumoto、Takahiro Kitagawa、Daisuke Imahori、Atsushi Matsuzaki、Youhei Saito、Tomoe Ohta、Tatsusada Yoshida、Yuji Nakayama、Eishi Ashihara、Tetsushi WatanabeDOI:10.1016/j.bmcl.2021.128161日期:2021.8inhibitor was isolated from the methanolic extract of the stems and twigs of Lindera umbellata together with epi-(-)-linderol A. Linderapyrone inhibited TCF/β-catenin transcriptional activity that was evaluated using cell-based TOPFlash luciferase assay system. To evaluate the structure-activity relationship and mechanism, we synthesized linderapyrone and its derivatives from piperitone. As the resultsLinderapyrone,一个的Wnt信号的抑制剂是从甲醇提取物中分离的茎和枝条乌药猪苓连同外延- ( - ) - linderol A. Linderapyrone抑制TCF / β-catenin 转录活性,使用基于细胞的 TOPFlash 荧光素酶检测系统进行评估。为了评估构效关系和机制,我们从胡椒酮合成了林萘吡酮及其衍生物。作为对合成化合物的进一步生物测定的结果,我们发现吡喃酮和单萜部分都是抑制作用所必需的。林德吡酮衍生物处理的人结肠直肠癌细胞中的 cDNA 微阵列分析表明,该化合物下调 Wnt 信号通路。此外,我们成功合成了比林德吡酮和ICG-001(阳性对照)具有更强抑制作用的林德吡酮衍生物。
-
Chemoselective transfer hydrogenation of α,β-unsaturated carbonyls catalyzed by a reusable supported Pd nanoparticles on biomass-derived carbon作者:Tao Song、Yanan Duan、Yong YangDOI:10.1016/j.catcom.2018.11.017日期:2019.2We herein report highly chemoselective transfer hydrogenation of α,β-unsaturated carbonyl compounds to saturated carbonyls with formic acid as a hydrogen donor over a stable and recyclable heterogeneous Pd nanoparticles (NPs) on N,O-dual doped hierarchical porous biomass-derived carbon. The synergistic effect between Pd NPs and incorporated heteroatoms on carbon plays a critical role on promoting the
-
Nanopalladium-catalyzed conjugate reduction of Michael acceptors – application in flow作者:Anuja Nagendiran、Henrik Sörensen、Magnus J. Johansson、Cheuk-Wai Tai、Jan-E. BäckvallDOI:10.1039/c5gc02920a日期:——A continuous-flow approach towards the selective nanopalladium-catalyzed hydrogenation of the olefinic bond in various Michael acceptors, which could lead to a greener and more sustainable process, has been developed. The...已经开发出一种连续流方法,用于在各种迈克尔受体中进行选择性纳米钯催化的烯烃键的加氢反应,这可能导致更绿色和更可持续的过程。这...
-
Catalytic Cross-Coupling of Alkylzinc Halides with α-Chloroketones作者:Chrysa F. Malosh、Joseph M. ReadyDOI:10.1021/ja0467768日期:2004.8.1this method, primary and secondary alkyl groups are introduced adjacent to a ketone carbonyl under mild reaction conditions and in good yield. Cyclic, acyclic, aromatic, and aliphatic α-chloroketones are suitable substrates. Optically active α-chloroketones are converted to optically active products. The reaction was found to proceed stereospecifically with inversion of stereochemistry. The reaction
-
COMBINATION PEPTIDE-NANOPARTICLES AND DELIVERY SYSTEMS INCORPORATING SAME申请人:Midatech Limited公开号:US20150099698A1公开(公告)日:2015-04-09Nanoparticles having a core and a corona of ligands covalently linked to the core, wherein differing species of peptides are bound to the nanoparticles and incorporated into various dosage forms.纳米颗粒具有核心和与核心共价连接的配体环,其中不同种类的肽与纳米颗粒结合,并纳入各种剂型中。
表征谱图
-
氢谱1HNMR
-
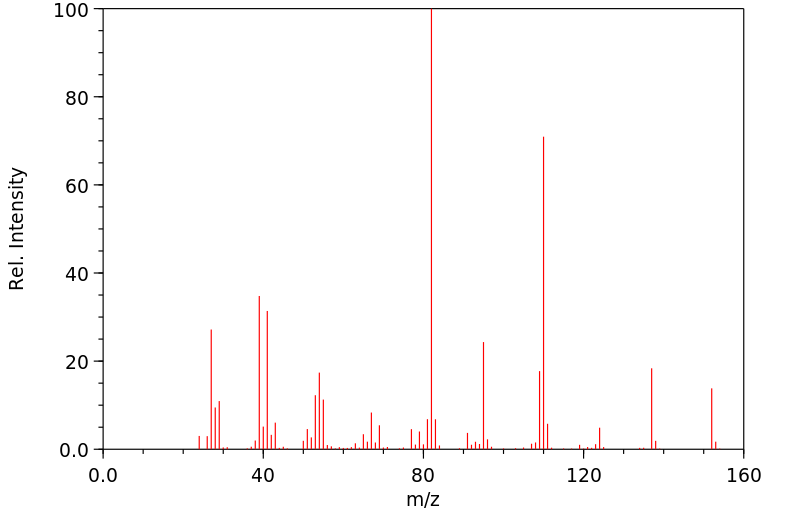
质谱MS
-
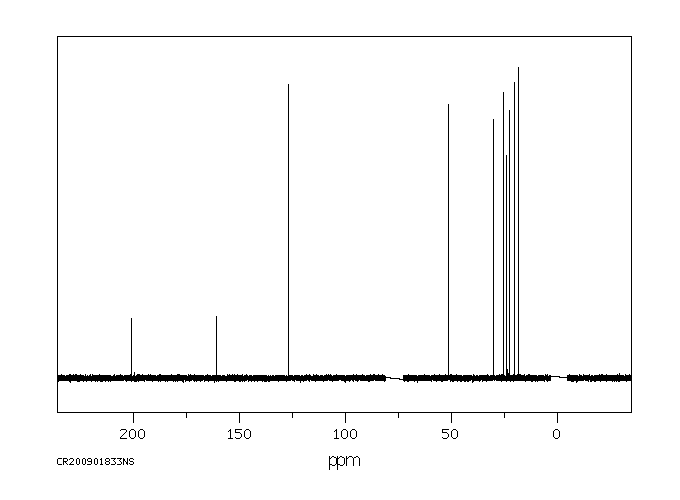
碳谱13CNMR
-
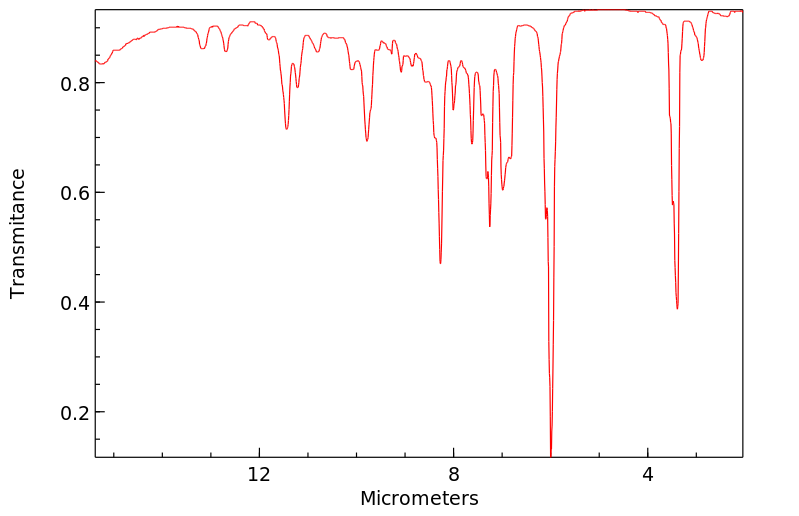
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸