桃金娘烯醛 | 564-94-3
中文名称
桃金娘烯醛
中文别名
6,6-二甲基二环[3.1.1]庚-2-烯-2-甲醛;(-)-桃金娘烯醛(香桃木醛)
英文名称
6,6-dimethylbicyclo[3.1.1]hept-2-ene-2-carbaldehyde
英文别名
myrtenal;6,6-dimethylbicyclo[3.1.1]hept-2-ene-2-carboxaldehyde
CAS
564-94-3
化学式
C10H14O
mdl
MFCD00137969
分子量
150.221
InChiKey
KMRMUZKLFIEVAO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:53-54 °C
-
沸点:220-221 °C(lit.)
-
密度:0.988 g/mL at 20 °C(lit.)
-
闪点:174 °F
-
溶解度:氯仿(微溶)、甲醇(微溶)
-
LogP:2.52
-
物理描述:Colourless liquid; refreshing, spicy-herbaceous odour
-
折光率:1.496-1.507
-
保留指数:1187;1175;1165;1169;1170;1176;1157;1165;1172;1168;1172;1179;1165;1165;1170;1165;1170;1164;1171;1166;1176;1165;1168;1188;1168;1151.46;1155.93;1160.56;1165.32;1170.25;1175.32;1180.54;1185.89;1191.36;1196.22;1173;1171;1173;1173;1169;1155;1169;1181;1169;1181.3;1153;1163;1172;1194;1172;1165;1163;1193;1153;1172;1173;1153;1174;1197;1158;1191;1167;1172;1165;1153;1168;1196;1171;1171;1165;1178;1159;1165;1181;1192;1174;1153;1173;1178;1180;1180;1166;1166;1188;1166;1162;1166;1165;1153;1175;1182;1173;1173;1165;1170;1175;1220;1176;1171;1173;1153;1174;1175;1165;1153;1171;1169;1169;1173
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:11
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.7
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
WGK Germany:2
-
RTECS号:DT5180000
-
安全说明:S23,S24/25
-
储存条件:室温
SDS
| Name: | (-)-Myrtenal 98% Material Safety Data Sheet |
| Synonym: | Benihinal; 6,6-Dimethylbicyclo(3.1.1)hept-2-ene-2-carboxaldehyde; 2-Formyl-6,6-dimethylbicyclo(3.1.1)hept-2-ene; Myrtenal; 2-Norpinene-2-carboxaldehyde, 6,6-dimethyl-; Bicyclo(3.1.1)hept-2-ene-2-carboxaldehyde, 6,6-dimethyl- |
| CAS: | 564-94-3 |
Synonym:Benihinal; 6,6-Dimethylbicyclo(3.1.1)hept-2-ene-2-carboxaldehyde; 2-Formyl-6,6-dimethylbicyclo(3.1.1)hept-2-ene; Myrtenal; 2-Norpinene-2-carboxaldehyde, 6,6-dimethyl-; Bicyclo(3.1.1)hept-2-ene-2-carboxaldehyde, 6,6-dimethyl-
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 564-94-3 | (-)-Myrtenal | 98 | 209-274-8 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea.
Inhalation:
May cause respiratory tract irritation.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Containers may explode when heated. Combustible liquid and vapor.
Extinguishing Media:
Use water spray to cool fire-exposed containers. Water may be ineffective. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Cover with sand, dry lime or soda ash and place in a closed container for disposal. Remove all sources of ignition.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use only in a well-ventilated area. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Keep away from heat and flame.
Storage:
Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate general or local explosion-proof ventilation to keep airborne levels to acceptable levels.
Exposure Limits CAS# 564-94-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear very slight yellow
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 220 - 221 deg C
Freezing/Melting Point: Not available.
Autoignition Temperature: Not applicable.
Flash Point: 78 deg C ( 172.40 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Not available.
Specific Gravity/Density: .9800 g/cm3
Molecular Formula: C10H14O
Molecular Weight: 150.22
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Ignition sources, excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong bases.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 564-94-3: DT5180000 LD50/LC50:
CAS# 564-94-3: Oral, rat: LD50 = 2300 mg/kg; Skin, rabbit: LD50 = >5 gm/kg.
Carcinogenicity:
(-)-Myrtenal - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 564-94-3: No information available.
Canada
CAS# 564-94-3 is listed on Canada's DSL List.
CAS# 564-94-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 564-94-3 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
蒎烯的一种,可应用于合成OLED和制造精油等领域。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-蒎烯聚合物 2,6,6-trimethylbicyclo[3.1.1]hept-2-ene 25766-18-1 C10H16 136.237 桃金娘烯醇 myrtenol 515-00-4 C10H16O 152.236 —— myrtenyl hydroperoxide 58434-29-0 C10H16O2 168.236 beta-蒎烯 β-pinene 127-91-3 C10H16 136.237 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 6,6-二甲基双环[3.1.1]庚-2-烯-2-乙醛 2-(6,6-Dimethylbicyclo<3.1.1>hept-2-ene)acetaldehyde 30897-75-7 C11H16O 164.247 —— 4-(6,6-dimethylbicyclo[3,1,1]hept-2-en-2-yl)-2-butanone 56933-99-4 C13H20O 192.301 6,6-二甲基双环[3.1.1]庚-2-烯-2-羧酸 myrtenic acid 19250-17-0 C10H14O2 166.22 6,6-二甲基双环[3.1.1]庚-2-烯-2-甲酰氯 myrtenyl chloride 146254-24-2 C10H13ClO 184.666 (1S)-2-异丙基-6,6-二甲基双环[3.1.1]庚-2-烯 (+)-2-Isopropylapopinene 156327-05-8 C12H20 164.291 —— (-)-2-Isopropylapopinene 923952-37-8 C12H20 164.291 2-乙烯基-6,6-二甲基双环[3.1.1]庚-2-烯 2-ethylene-6,6-dimethylbicyclo<3.1.1>hept-2-ene 473-00-7 C11H16 148.248 —— myrtenyl bromide 22339-11-3 C10H15Br 215.133 桃金娘烯醇 myrtenol 515-00-4 C10H16O 152.236 7,7-二甲基双环[3.1.1]庚-3-烯 Apopinen 32863-61-9 C9H14 122.21 —— 1-(6,6-dimethyl-bicyclo[3.1.1]hept-2-en-2-yl)-ethanol 33829-99-1 C11H18O 166.263 —— E-(dimethyl-6,6-bicyclo<3.1.1>-hepten-yl-3)-3-methyl-2-propen-2-oate de methyle 89996-95-2 C14H20O2 220.312 —— myrtenal thiosemicarbazone —— C11H17N3S 223.342 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:在乙醚/甲醇/五氟苯酚溶剂体系中用硼氢化钠还原α,β-不饱和羰基化合物。N,N,N',N'-四甲基乙二胺和1-己烯作为硼烷清除剂的用途摘要:五氟苯酚可作为有效的质子源,用于还原含甲醇的乙醚中的硼氢化钠。该系统允许以高收率将α,β-不饱和羰基化合物区域选择性还原为相应的烯丙基醇。使用1-己烯或N,N,N',N'-四甲基乙二胺作为硼烷清除剂可适度改善该反应的区域选择性。幸运的是,原位生成的三五氟苯氧基硼氢化钠可产生α,β-不饱和羰基化合物的区域特异性1,2-还原。DOI:10.1016/0022-1139(93)05005-l
-
作为产物:参考文献:名称:A Metallomicellar Catalyst for Controlled Oxidation of Alcohols and Lignin Mimics in Water using Open Air as Oxidant摘要:摘要 醇基和 β-O-4 (C-C) 链广泛存在于生物质原料中,它们是丰富的可再生增值化学品资源。利用露天空气作为氧化剂,以可控方式开发原料材料的直接氧化或氧化裂解的可持续方案,是生产具有重要工业价值的羰基化合物的一项具有启发性的任务。此外,将木质素氧化解聚成精细化学品近来也引起了人们的兴趣。在此,我们报告了首个催化剂系统实例,该催化剂系统可激活大气中的分子氧,在露天条件下对醇类、β-O-4(C-C)连接和水中真正的木质素等原料进行受控氧化和氧化裂解/解聚。羰基产物的选择性可通过在 ~7.0 和 ~12.0 之间改变 pH 值来控制。目前的策略突出了不使用任何外部助催化剂、氧化剂、自由基添加剂和/或破坏性有机溶剂的特点。该催化剂具有广泛的底物范围和出色的官能团耐受性。使用廉价的催化剂和易于获得的氧化剂进行多克级合成,证明了所开发方案的实用性。在一些对照实验、动力学和计算研究的帮助下,我们提出了一种合理的机制。DOI:10.1002/cssc.202301754
文献信息
-
Wittig Reactions of Trialkylphosphine-derived Ylides: New Directions and Applications in Organic Synthesis作者:James McNulty、David McLeod、Priyabrata Das、Carlos Zepeda-VelázquezDOI:10.1080/10426507.2014.980907日期:2015.6.3ylides derived from short-chain trialkylphosphines in the Wittig-type olefination reactions toward the synthesis of alkenes, including stilbenes, styrenes, and 1,3-dienes, as well as reagents for homologation reactions, are described. The methods allow easy access to alkenes with high (E)-stereoselectivity in good yield. These reactions are conducted with weak bases in aqueous media, which allows easy
-
Sulfur-controlled and rhodium-catalyzed formal (3 + 3) transannulation of thioacyl carbenes with alk-2-enals and mechanistic insights作者:Qiuyue Wu、Ziyang Dong、Jiaxi Xu、Zhanhui YangDOI:10.1039/d1ob00116g日期:——
A rhodium-catalyzed denitrogenative formal (3 + 3) transannulation of 1,2,3-thiadiazoles with alk-2-enals is achieved and a mechanistic investigation is performed, with an inverse KIE of 0.49 obtained.
-
A new synthesis of α,β-unsaturated aldehydes作者:Jean-Marc Nuzillard、Ahcene Boumendjel、Georges MassiotDOI:10.1016/s0040-4039(01)80653-5日期:1989.1The two carbon homologation of carbonyl compounds to α,β-unsaturated aldehydes is achieved by the Wittig-Horner reaction with N-methoxy N-methyl diethylphosphonoacetamide followed by lithium aluminum hydride reduction.
-
Pharmaceutical Compositions Comprising Deuterium-Enriched Perillyl Alcohol, Iso-Perillyl Alcohol and Derivatives Thereof申请人:NEONC TECHNOLOGIES INC.公开号:US20160039731A1公开(公告)日:2016-02-11The present invention, provides for a deuterium-enriched monoterpene or sesquiterpene such as perillyl alcohol, or a deuterium-enriched isomer or analog of monoterpenes or sesquiterpenes, such as isoperillyl alcohol. The present invention also provides for a deuterium-enriched derivative of a monoterpene or sesquiterpene, such as a perillyl alcohol carbamate or a deuterium-enriched derivative of an isomer or analog of a monoterpene or sesquiterpene, such as an isoperillyl alcohol carbamate. The deuterium-enriched derivative may be perillyl alcohol or isoperillyl alcohol conjugated with a therapeutic agent such as a chemotherapeutic agent. The present invention also provides for a method of treating a disease such as cancer, comprising the step of delivering to a patient a therapeutically effective amount of a deuterium-enriched compound.
-
[EN] METHODS AND DEVICES FOR USING ISOPERILLYL ALCOHOL<br/>[FR] PROCÉDÉS ET DISPOSITIFS POUR L'UTILISATION D'ALCOOL ISOPERILLYLIQUE申请人:NEO ONCOLOGY INC公开号:WO2012083178A1公开(公告)日:2012-06-21The present invention provides for a method of treating a disease such as cancer, comprising the step of administering to a patient a therapeutically effective amount of an isomer or analog of monoterpene or sesquiterpene (or its derivative), such as an isoperillyl alcohol. The present invention also provides for a method of treating a disease comprising the step of administering to a patient a therapeutically effective amount of a derivative of an isomer or analog of monoterpene or sesquiterpene, such as an isoperillyl alcohol carbamate. The derivative may be an isoperillyl alcohol conjugated with a therapeutic agent such as a chemotherapeutic agent. The route of administration may vary, including inhalation, intranasal, oral, transdermal, intravenous, subcutaneous or intramuscular injection.
表征谱图
-
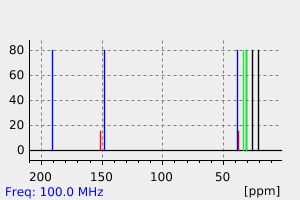
氢谱1HNMR
-
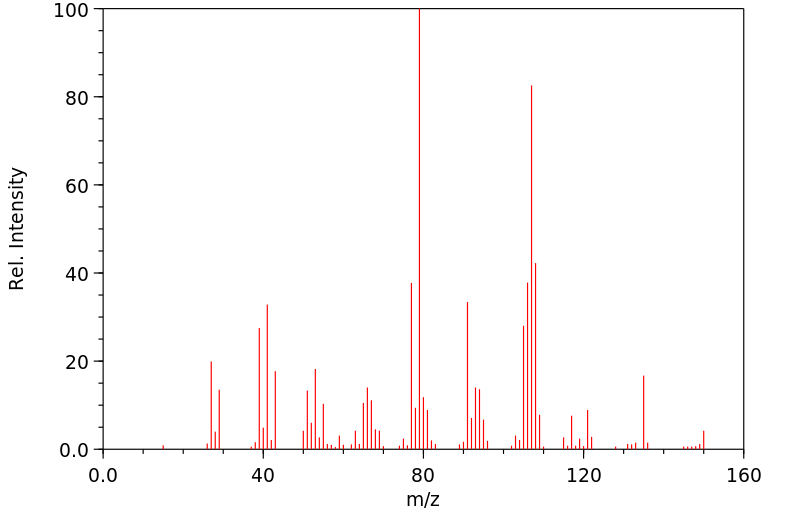
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸