γ-丁内酯 | 96-48-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
稳定性/保质期:
化学性质:该物质在热碱作用下易发生水解,水解是可逆的,在pH=7时又生成内酯;在酸性介质中水解较慢。
-
本品属低毒类物质,对中枢神经有麻醉作用。大鼠经口LD₅₀=1800 mg/kg,对皮肤有刺激作用,其烟雾可刺激眼睛、黏膜和上呼吸道。
-
存在于烤烟、白肋烟、香料烟的烟叶中,也存在于烟气中。
-
还存在于杏、面包和咖啡中,并且是炒榛子挥发性香气成分之一。
-
制备方法如下:在反应瓶中加入干燥乙腈2 mL,三甲基氯硅烷110 mg(1.0 mmol),氮气保护下于0 ℃加入硝酸银187 g(1.1 mmol)。搅拌反应1小时后倾出上清液(除去氯化银),在搅拌下加至CrO₃ 150 mg(1.5 mmol)的1 mL乙腈中,搅拌反应15分钟。然后,在冰水浴冷却下慢慢滴加THF(2)(1.0 mmol)溶于1 mL乙腈中的溶液,室温搅拌反应24小时。过滤、二氯甲烷洗涤后蒸出溶剂,得到粗品①(1)。硅胶柱纯化后收率为65%。注:该反应可能的机理如下。(参考书494页)[1]
-
计算性质
-
辛醇/水分配系数(LogP):-0.6
-
重原子数:6
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
ADMET
制备方法与用途
-
顺酐加氢法:该法是70年代发展的先进工艺,通过一段加氢反应,能以任意比例生产四氢呋喃和γ-丁内酯。通常的比例是四氯呋喃:γ-丁内酯=3-4:1。生产企业较多但规模较小,平均年产量约为300吨。该法占国内总产能的30%。
-
1,4-丁二醇脱氢法:反应器为列管式,装填片状铜催化剂(以氧化锌为载体)。反应温度控制在230-240℃。反应产物粗γ-丁内酯经减压蒸馏得成品,收率可达77%以上。
-
1,4-丁二醇制备法:以1,4-丁二醇为原料,先预热,与氢气在铜催化剂存在下反应,温度控制在230~240℃,得到γ-丁内酯粗品,经减压蒸馏得产品。
-
烯丙醇法制备法:以芳烃为溶剂、铑络合物为催化剂,将烯丙醇与原料气(H2+CO)醛化,以骨架镍催化加氢得1,4-丁二醇,进而制得γ-丁内酯并联产四氢呋喃。
-
顺酐加氢法:该工艺是70年代发展的先进技术,通过一次加氢反应能任意比例生产四氢呋喃和γ-丁内酯。常用的比例为四氯呋喃:γ-丁内酯=3-4:1。生产企业较多但规模小,平均年产量约为300吨。该方法占国内总产能的30%。
-
1,4-丁二醇脱氢法:反应器设计为列管式,填充片状铜催化剂(以氧化锌为载体)。控制反应温度在230-240℃之间。粗γ-丁内酯经减压蒸馏后获得成品,收率通常超过77%。
-
1,4-丁二醇制备法:利用1,4-丁二醇作为原料,先预热,与氢气在铜催化剂存在下反应,温度控制在230~240℃之间,产生γ-丁内酯粗品。然后通过减压蒸馏得到产品。
-
烯丙醇法制备法:使用芳烃作为溶剂和铑络合物作为催化剂,将烯丙醇与原料气(H2+CO)醛化,通过骨架镍催化加氢得1,4-丁二醇。进而制得γ-丁内酯并联产四氢呋喃。
γ-丁内酯主要用于气相色谱固定液,最高使用温度为30℃,溶剂采用甲醇。它能够分离和分析烃类及各种含氧化合物、永久性气体;合成聚乙烯吡咯烷酮、DL-甲硫氨酸、六氢吡啶、苯丁酸和硫代乙酸等中间体;作为聚丙烯腈、乙酸纤维、聚甲基丙烯酸甲酯和聚苯乙烯的溶剂。此外,它还具有良好的抗氧性、增塑性、萃取性、吸收性、分散性和固色作用,在医药行业中可作麻醉剂及镇静剂,并可用于合成环丙沙星和干扰素等药物;在农林业中是植物生长剂和杀虫剂的中间体;也可用于电池、电容器制作,彩卷成色等。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 丁二酸酐 succinic acid anhydride 108-30-5 C4H4O3 100.074 丁酸甲酯 Methyl butyrate 623-42-7 C5H10O2 102.133 4-羟基丁酸甲酯 methyl 4-hydroxybutanoate 925-57-5 C5H10O3 118.133 —— 4-hydroxybutyl 4-hydroxybutanoate —— C8H16O4 176.213 —— 4-hydroxybutanoic acid lactone 50768-69-9 C4H6O3 102.09 4-氯丁酸乙酯 4-chloro-butyric acid ethyl ester 3153-36-4 C6H11ClO2 150.605 4-溴丁酸乙酯 4-bromoethylbutanoate 2969-81-5 C6H11BrO2 195.056 丁二酸二甲酯 Dimethyl succinate 106-65-0 C6H10O4 146.143 4-碘-丁酸乙酯 ethyl 4-iodobutyrate 7425-53-8 C6H11IO2 242.057 丁二酸二乙酯 succinic acid diethyl ester 123-25-1 C8H14O4 174.197 4-溴丁酸甲酯 Methyl 4-bromobutyrate 4897-84-1 C5H9BrO2 181.029 4-氨基丁酸甲酯 methyl 4-aminobutyrate 3251-07-8 C5H11NO2 117.148 丁酸 butyric acid 107-92-6 C4H8O2 88.1063 琥珀半醛 Succinic semialdehyde 692-29-5 C4H6O3 102.09 4-羟基丁酸 4-hydroxybutanoic acid 591-81-1 C4H8O3 104.106 丙酸烯丙酯 prop-2-enyl propanoate 2408-20-0 C6H10O2 114.144 丁二酸二丁酯 dibutyl succinate 141-03-7 C12H22O4 230.304 β-丙内酯 β-Propiolactone 57-57-8 C3H4O2 72.0636 戊二酸酐 glutaric anhydride, 108-55-4 C5H6O3 114.101 —— 5-Chloro-dihydro-2(3H)-furanone 36603-83-5 C4H5ClO2 120.535 α-溴-γ-丁内酯 α-bromo-γ-butyrolactone 5061-21-2 C4H5BrO2 164.986 - 1
- 2
- 3
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 丁酸乙酯 Ethyl butyrate 105-54-4 C6H12O2 116.16 丁酸甲酯 Methyl butyrate 623-42-7 C5H10O2 102.133 4-羟基丁酸甲酯 methyl 4-hydroxybutanoate 925-57-5 C5H10O3 118.133 乙酰乙酸甲酯 4-oxobutanoic acid methyl ester 13865-19-5 C5H8O3 116.117 4-羟基丁酸乙酯 ethyl 4-hydroxybutanoate 999-10-0 C6H12O3 132.159 二氢-4-甲基 2(3H)-呋喃酮 dihydro-4-methyl-2(3H)-furanone 1679-49-8 C5H8O2 100.117 α-甲基-γ-丁内酯 3-methyltetrahydro-2-furanone 1679-47-6 C5H8O2 100.117 —— (3S)-3-methyldihydrofuran-2(3H)-one 65527-79-9 C5H8O2 100.117 4-甲氧基丁酸甲酯 Methyl 4-methoxybutyrate 29006-01-7 C6H12O3 132.159 —— 4-Formyloxy-buttersaeure 89364-40-9 C5H8O4 132.116 —— 4-hydroxybutyl 4-hydroxybutanoate —— C8H16O4 176.213 乙基-4-乙氧基丁酸 4-ethoxy-butyric acid ethyl ester 26448-91-9 C8H16O3 160.213 醋羟丁酸 4-acetoxybutyric acid 26976-72-7 C6H10O4 146.143 —— n-Propyl 4-n-propoxybutanoate 155060-42-7 C10H20O3 188.267 —— methyl 4-(acetyloxy)butanoate 25560-92-3 C7H12O4 160.17 4-甲氧基丁酸 4-methoxybutyric acid 29006-02-8 C5H10O3 118.133 4-溴丁酸乙酯 4-bromoethylbutanoate 2969-81-5 C6H11BrO2 195.056 4-氯丁酸乙酯 4-chloro-butyric acid ethyl ester 3153-36-4 C6H11ClO2 150.605 丁二酸二甲酯 Dimethyl succinate 106-65-0 C6H10O4 146.143 —— n-propyl 4-bromobutanoate 4890-39-5 C7H13BrO2 209.083 4-氯丁酸丙基酯 4-chloro-butyric acid propyl ester 3153-35-3 C7H13ClO2 164.632 4-乙酰氧基丁酸乙酯 ethyl 4-(acetyloxy)butanoate 25560-91-2 C8H14O4 174.197 4-碘-丁酸乙酯 ethyl 4-iodobutyrate 7425-53-8 C6H11IO2 242.057 (±)-α-羟基-γ-丁内酯 3-hydroxyoxolan-2-one 19444-84-9 C4H6O3 102.09 丁酸 butyric acid 107-92-6 C4H8O2 88.1063 4-溴丁酸甲酯 Methyl 4-bromobutyrate 4897-84-1 C5H9BrO2 181.029 4-氯丁酸甲酯 methyl 4-chlorobutyrate 3153-37-5 C5H9ClO2 136.578 4-碘丁酸甲酯 methyl 4-iodobutanoate 14273-85-9 C5H9IO2 228.03 4-羟基丁酸 4-hydroxybutanoic acid 591-81-1 C4H8O3 104.106 —— 4-ethoxybutanoic acid 10374-37-5 C6H12O3 132.159 —— β-Fluoro-γ-butyrolactone —— C4H5FO2 104.081 4-溴丁酸丁酯 butyl 4-bromobutanoate 3540-75-8 C8H15BrO2 223.11 4-氯丁酸丁酯 butyl 4-chlorobutyrate 3153-33-1 C8H15ClO2 178.659 5-溴二氢呋喃-2(3H)-酮 α-bromo-γ-butyrolactone 94386-29-5 C4H5BrO2 164.986 —— allyl 4-hydroxybutanoate 285982-03-8 C7H12O3 144.17 —— 5-oxa-nonanedioic acid 7423-25-8 C8H14O5 190.196 3-氯二氢呋喃-2(3H)-酮 α-chloro-γ-butyrolactone 31167-90-5 C4H5ClO2 120.535 3-氟二氢呋喃-2(3H)-酮 2,3-dideoxy-2-fluoro-D,L-glycero-tetronolactone 3885-31-2 C4H5FO2 104.081 —— γ-Fluoro-γ-butyrolactone 2343-90-0 C4H5FO2 104.081 2-甲烯基丁内酯 α-methylene-γ-butyrolactone 547-65-9 C5H6O2 98.1014 乙酰乙酸乙酯 ethyl acetoacetate 141-97-9 C6H10O3 130.144 —— 4-hydroxybutanoic acid, n-decyl ester —— C14H28O3 244.375 α-溴-γ-丁内酯 α-bromo-γ-butyrolactone 5061-21-2 C4H5BrO2 164.986 - 1
- 2
- 3
- 4
- 5
反应信息
-
作为反应物:参考文献:名称:从环烷基胺生物合成尼龙单体的集成辅因子/副产物回收级联反应摘要:我们报道了一种高度原子高效的辅因子/副产品回收级联反应,采用环烷基胺作为合成尼龙构件的多方面原料。使用大肠杆菌全细胞以及纯化的酶进行的反应可在不同的底物浓度下分别产生大于80%和95%的所需ω-氨基酸的出色转化率。通过使用工程化的生物催化剂证明了该串联生物催化级联的适用性以产生相应的内酰胺。例如,通过使用全细胞生物催化剂,由10 mM环己胺合成了有价值的聚合物β-己内酰胺,其转化率为75%。这种级联反应可以替代基于生物的ω-氨基酸和相应内酰胺化合物的生产。DOI:10.1002/anie.202012658
-
作为产物:描述:丁二酸酐 在 tris(triphenylphosphine)ruthenium(II) chloride 氢气 作用下, 以 various solvent(s) 为溶剂, 170.0 ℃ 、100.0 kPa 条件下, 生成 γ-丁内酯参考文献:名称:钌配合物催化新型制备γ-丁内酯摘要:γ-丁内酯(以下简称GBL)是通过液相进行马来酸酐(MAH)的两步加氢而制得的:第一步是将MAH加氢为琥珀酸酐(SAH),然后将SAH加氢为GBL。第二阶段。已经使用均相催化剂研究了后者的氢化。开发了由Ru(acac)3,P(辛基)3和对甲苯磺酸(p -TsOH)组成的新型钌催化剂体系,用于SAH的加氢反应,具有优异的催化性能,对GBL的选择性超过95%以上活性高于文献报道。发现p-TsOH不仅在提高反应速率方面而且在提高选择性方面都起着重要作用。对-TsOH诱导Ru络合物发生结构变化,导致阳离子变化,从而显示出更高的催化剂活性。它也防止了由游离P(辛基)3催化的不希望的副反应,从而导致对GBL的高选择性。研究了生产GBL的方法。该方法的一些新颖特征包括Ru配合物的外部制备方法,偶联反应以及分离以去除H 2 O(SAH的氢化产物)以提高反应速率的方法。还开发了催化剂回收系统以回收超过90%的催化剂。DOI:10.1006/jcat.2000.2945
-
作为试剂:描述:4,4’-二酰氯二苯醚 、 N-(2-氨基-4-硝基苯基)-n-苯胺 在 三乙胺 、 γ-丁内酯 、 对甲苯磺酸 作用下, 以 四氢呋喃 为溶剂, 反应 22.0h, 以81.5%的产率得到2,2'-(4,4'-oxybiphenyl)bis(1-phenyl-5-nitrobenzimidazole)参考文献:名称:一种含双N-取代苯并咪唑二胺及其制备方法和应用摘要:本发明提供了一种含双N‑取代苯并咪唑二胺及其制备方法和应用,属于有机合成技术领域。本发明提供的含双N‑取代苯并咪唑二胺中双苯并咪唑环都带有侧基,双N‑取代苯并咪唑的引入在保留传统苯并咪唑端氨基较强亲和性的同时,可以增加该类分子侧基的空间位阻效应,进而提高利用本发明的二胺单体制备的聚合材料的热学性能和溶解性;此外,本发明在双N‑取代苯并咪唑环之间引入了柔性基团或大体积侧基,进一步增加该类分子的柔性和空间位阻效应,进一步提高本发明的二胺单体制备的聚合材料的溶解性。公开号:CN112778211A
文献信息
-
SYNTHESIS OF CYCLIC AMIDINES申请人:Lentzen George公开号:US20110178292A1公开(公告)日:2011-07-21The invention relates to an innovative method for synthesis of cyclic amidines. The synthesis starts from a β-, γ- or δ-lactone which is twofold brominated. After esterification of the carboxyl function, the bromine atoms are nucleophilically substituted and the corresponding diamino compound is obtained. The ring closure to the cyclic amidine is accomplished subsequently by reaction with orthoester, imidate or thioimidate. Owing to interposing additional steps for recovery of the diamino compound in enantiomerically pure form, the enantiomers of the cyclic amidines can be stereoselectively synthesized.
-
Total Synthesis of (−)-21-Isopentenylpaxilline作者:Amos B. Smith、Haifeng CuiDOI:10.1021/ol027575g日期:2003.2.1[structure: see text] The total synthesis of (-)-21-isopentenylpaxilline (1) has been achieved. Key elements of the synthesis include the stereocontrolled construction of the advanced eastern hemisphere (-)-5, a highly efficient union of the eastern and western fragments (-)-5 and 4, respectively, exploiting our 2-substituted indole synthesis, and a new protocol for the construction of ring C.
-
Metal‐Free Synthesis of <i>N</i> ‐Aryl Amides using Organocatalytic Ring‐Opening Aminolysis of Lactones作者:Wusheng Guo、José Enrique Gómez、Luis Martínez‐Rodríguez、Nuno A. G. Bandeira、Carles Bo、Arjan W. KleijDOI:10.1002/cssc.201700415日期:2017.5.9Catalytic ring‐opening of bio‐sourced non‐strained lactones with aromatic amines can offer a straightforward, 100 % atom‐economical, and sustainable pathway towards relevant N‐aryl amide scaffolds. Herein, the first general, metal‐free, and highly efficient N‐aryl amide formation is reported from poorly reactive aromatic amines and non‐strained lactones under mild operating conditions using an organic
-
Reactions of methyl anthranilate with ethyl γ-bromobutyrate作者:I. McCall、G. R. Proctor、L. PurdieDOI:10.1039/j39700001126日期:——When methyl anthranilate and ethyl γ-bromobutyrate are heated together, various products are formed: above 125° the main product is a yellow compound for which a 7b,12b-diazabenz[e]aceanthrylene structure is postulated.
-
A facile two synthon approach to the camptothecin skeleton作者:H.G.M. Walraven、U.K. PanditDOI:10.1016/0040-4020(80)80022-6日期:1980.1A synthesis of deethyldesoxycamptothecin via the reaction of two readily accessible synthons is described. One of the synthons constitutes the ABC ring system of camptothecin, while the second provides all the C atoms of the rings D and E. The synthetic approach is suited for the total synthesis of camptothecin analogues.
表征谱图
-
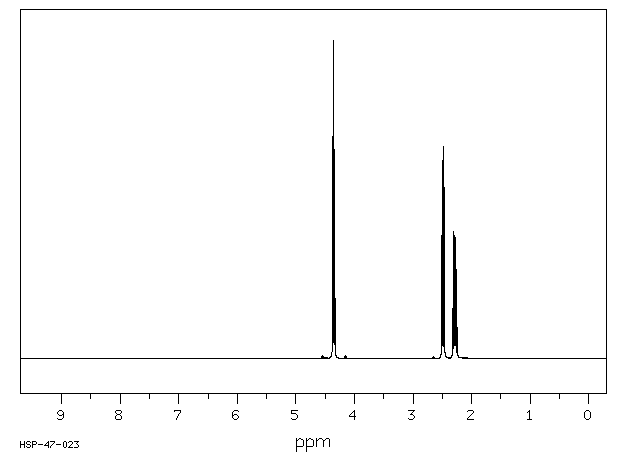
氢谱1HNMR
-
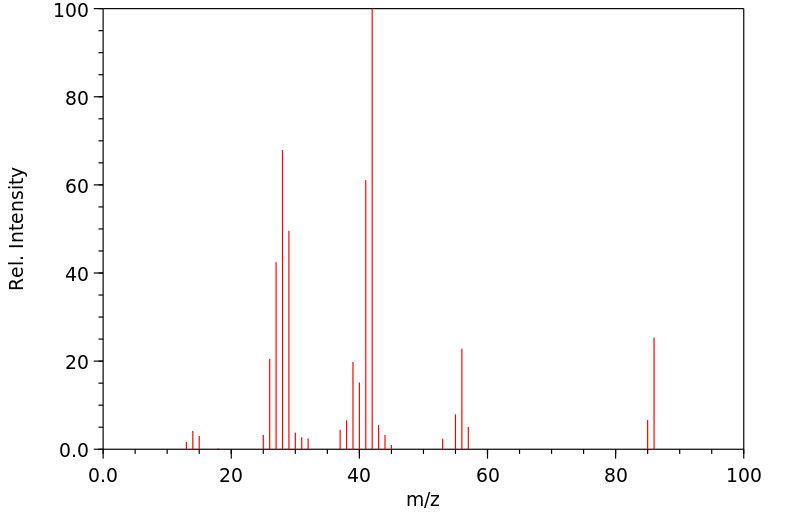
质谱MS
-
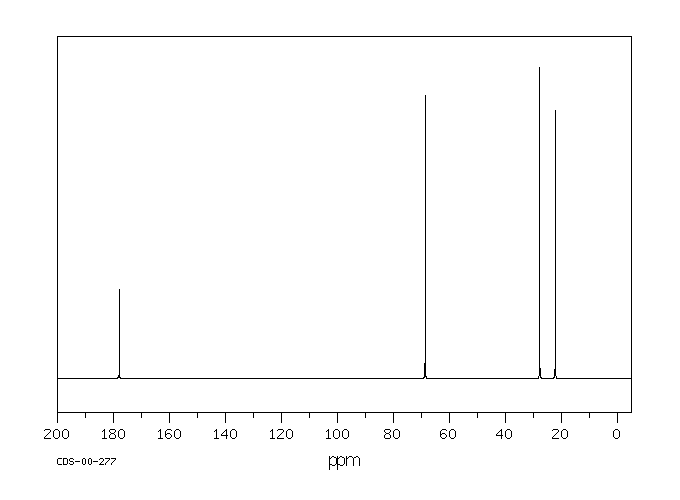
碳谱13CNMR
-
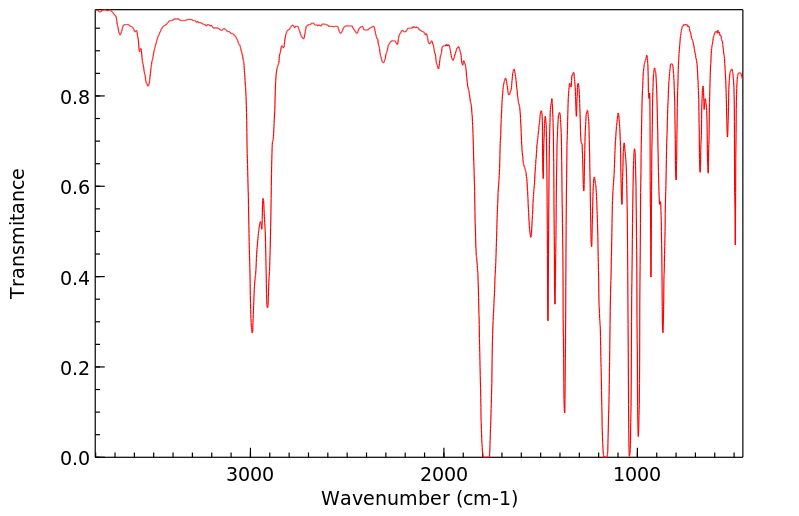
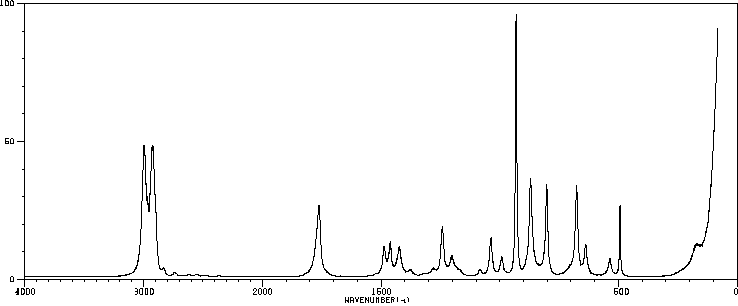
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息