对甲苯磺酸 | 104-15-4
物质功能分类
中文名称
对甲苯磺酸
中文别名
4-甲苯磺酸;甲苯磺酸;对甲基苯磺酸;4-甲基苯磺酸;甲苯-4-磺酸
英文名称
toluene-4-sulfonic acid
英文别名
p-toluenesulphonic acid monohydrate;para-toluenesulphonic acid;Toluene-p-sulfonic acid;p-Toluenesulfonic acid;p-tolylsulfonic acid;pTSA;TsOH;p-TsOH;4-methylbenzenesulfonic acid;p-toluenesulfonic acid monohydrate;4-methylbenzene-1-sulfonic acid;para-toluenesulfonic acid;p-toluenesulphonic acid;4-toluenesulfonic acid;tosic acid;p-toluenesulfonic acid hydrate;p‐toluenesulfonic acid;p-methyl benzenesulfonic acid;toluenesulfonic acid;tosylic acid;Hydron;4-methylbenzenesulfonate
CAS
104-15-4
化学式
C7H8O3S
mdl
MFCD00064387
分子量
172.205
InChiKey
JOXIMZWYDAKGHI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:106~107℃
-
沸点:116 °C
-
密度:1.07
-
闪点:41 °C
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
-
LogP:0.41 at 25℃
-
物理描述:Toluene sulfonic acid, liquid, with more than 5% free sulfuric acid appears as colorless to black solid. Odorless or nearly odorless. (USCG, 1999)
-
颜色/状态:MONOCLINIC LEAFLETS OR PRISMS
-
蒸汽压力:0.0000027 [mmHg]
-
稳定性/保质期:
-
解离常数:-1.34
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:62.8
-
氢给体数:1
-
氢受体数:3
ADMET
代谢
产生3-甲基邻苯二酚的假单胞菌:Focht, DD & Williams, FD,《加拿大微生物学杂志》,16卷,309页(1970年);产生4-甲基邻苯二酚的假单胞菌:Cain, RB & Farr, DR,《生物化学杂志》,106卷,859页(1968年)。/来自表格/
YIELDS 3-METHYLCATECHOL IN PSEUDOMONAS: FOCHT, DD & WILLIAMS, FD, CAN J MICROBIOL, 16, 309 (1970); YIELDS 4-METHYLCATECHOL IN PSEUDOMONAS: CAIN, RB & FARR, DR, BIOCHEM J. 106, 859 (1968). /FROM TABLE/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
所有暴露途径均可能产生严重的局部影响。
Serious local effects by all routes of exposure.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
喉咙痛。咳嗽。灼热感。呼吸困难。气短。
Sore throat. Cough. Burning sensation. Laboured breathing. Shortness of breath.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
红肿。疼痛。严重皮肤烧伤。
Redness. Pain. Serious skin burns.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
红斑。疼痛。烧伤。
Redness. Pain. Burns.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
喉咙痛。喉咙和胸部有灼热感。休克或晕厥。
Sore throat. Burning sensation in the throat and chest. Shock or collapse.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
吸收、分配和排泄
很可能会以对甲苯磺酸的形态被排出。/来自表格/
PROBABLY EXCRETED AS P-TOLUENESULFONIC ACID. /FROM TABLE/
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
经口服或肠道外给药后,对甲苯磺酸盐在组织中均匀分布,除了大脑和脂肪组织。
FOLLOWING ORAL OR IP ADMIN, P-TOLUENESULFONATE WAS EVENLY DISTRIBUTED IN TISSUES, EXCEPT IN BRAIN & FAT TISSUES.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
危险等级:8
-
危险品标志:C
-
安全说明:S23,S26,S45
-
危险类别码:R34,R10
-
海关编码:2904909090
-
危险品运输编号:2585
-
危险类别:8
-
RTECS号:XT6300000
-
包装等级:III
-
储存条件:密封保存,存放在阴凉、干燥的地方,并远离火源。建议使用塑料袋外加木箱包装,然后存放于阴凉、通风、干燥处,并按照有毒化学品的规定进行储运。
SDS
| Name: | p-Toluenesulfonic Acid Monohydrate Material Safety Data Sheet |
| Synonym: | Benzenesulfonic acid, 4-methyl-, monohydrate; Toluene, 4-sulfonic acid, monohydrate; PTSA monohydrate |
| CAS: | 104-15-4 |
Synonym:Benzenesulfonic acid, 4-methyl-, monohydrate; Toluene, 4-sulfonic acid, monohydrate; PTSA monohydrate
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 104-15-4 | p-Toluenesulfonic acid monohydrate | > 97.5 | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.Hygroscopic (absorbs moisture from the air).
Potential Health Effects
Eye:
Causes severe eye irritation and possible burns.
Skin:
Causes skin irritation. Not expected to cause an allergic skin reaction.
Ingestion:
May be harmful if swallowed.
Inhalation:
Dust is irritating to the respiratory tract.
Chronic:
Chronic exposure may cause effects similar to those of acute exposure.
Section 4 - FIRST AID MEASURES
Eyes: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid immediately.
Skin:
In case of contact, flush skin with plenty of water. Remove contaminated clothing and shoes. Get medical aid if irritation develops and persists. Wash clothing before reuse.
Ingestion:
If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. This material in sufficient quantity and reduced particle size is capable of creating a dust explosion. Contact with metals may evolve flammable hydrogen gas.
Extinguishing Media:
Use water spray, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Keep container tightly closed. Avoid breathing dust. Do not get in eyes. Avoid contact with skin and clothing.
Storage:
Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 104-15-4: CAS# 6192-52-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystals
Color: white
Odor: none reported
pH: Not applicable.
Vapor Pressure: 0.0000027 mm Hg @ 25 deg C
Viscosity: Not available.
Boiling Point: 140 deg C @ 20 mmHg
Freezing/Melting Point: 103 deg C
Autoignition Temperature: 350 deg C ( 662.00 deg F)
Flash Point: 180 deg C ( 356.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Soluble.
Specific Gravity/Density: Not available.
Molecular Formula: C7H8O3S.H2O
Molecular Weight: 190.22
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Dust generation, moisture, excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong bases, metals.
Hazardous Decomposition Products:
Carbon monoxide, oxides of sulfur, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 104-15-4: XT6300000 CAS# 6192-52-5: DB7164000 LD50/LC50:
CAS# 104-15-4: Oral, rat: LD50 = 2480 mg/kg.
CAS# 6192-52-5: Oral, mouse: LD50 = 1683 mg/kg; Oral, rat: LD50 = 2570 mg/kg.
Skin absorption LD50 guinea pig: no evidence at 1.0 g/kg.
irritation, guinea pig: Moderate.
pig: Moderate exacerbation.
sensitize any animals tested.
Company) Carcinogenicity:
p-Toluenesulfonic acid anhydrous - Not listed by ACGIH, IARC, or NTP.
p-Toluenesulfonic acid monohydrate - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: Fathead Minnow: > 100 mg/l; 96 hr; LC50
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: ARYLSULPHONIC ACIDS, SOLID
Hazard Class: 8
UN Number: 2585
Packing Group: III
IMO
Shipping Name: ARYLSULPHONIC ACIDS, SOLID
Hazard Class: 8
UN Number: 2585
Packing Group: III
RID/ADR
Shipping Name: ARYLSULPHONIC ACIDS, SOLID
Hazard Class: 8
UN Number: 2585
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37 Wear suitable gloves.
WGK (Water Danger/Protection)
CAS# 104-15-4: 1
CAS# 6192-52-5: 1
Canada
CAS# 104-15-4 is listed on Canada's DSL List.
CAS# 104-15-4 is not listed on Canada's Ingredient Disclosure List.
CAS# 6192-52-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 104-15-4 is listed on the TSCA inventory.
CAS# 6192-52-5 is not on the TSCA Inventory because it is a hydrate.
It is considered to be listed if the CAS number for the anhydrous form
is on the inventory (40CFR720.3(u)(2)).
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
概述
化学性质
对甲苯磺酸(分子结构式:p-CH₃C₆H₄SO₃H,也写作TsOH,英文P-Toluene Sulfonic acid),简称PTS,是一个不具氧化性的有机强酸。它为白色针状或粉末状结晶,可溶于水、醇、醚和其他极性溶剂。对甲苯磺酸极易潮解,易使木材和棉织物脱水而碳化,难溶于苯和甲苯;在碱熔时会生成对甲酚。
用途对甲苯磺酸广泛应用于医药合成、农药制造、聚合反应的稳定剂以及有机合成(如酯类等)的催化剂。它还是涂料中间体和树脂固化剂。对甲苯磺酸是常用的有机酸催化剂,用于制备对甲苯磺酸钠,并进一步与五氯化磷作用生成对甲苯磺酰氯,后者在亲核取代反应中发挥作用。
制备工业上通过使用浓硫酸对甲苯进行磺化来制取对甲基苯磺酸。制得的对甲苯磺酸中可能含有少量苯磺酸和硫酸杂质,可通过将不纯样品置于浓盐酸中重结晶并共沸干燥以得到纯净产品。
参考质量标准| 项目/指标 | 工业级 | 医药级 | 精制级 | 试剂级(化学纯) |
|---|---|---|---|---|
| 含量(以C₇H₈O₃S·H₂O计,%)≥ | 90-93.0 | 96.0 | 97.0 | 98.0 |
| 游离酸(H₂SO₄,%)≤ | 3.0 | 0.7 | 0.5 | 0.1 |
| 水分(不包括结晶水,%)≤ | 4.0 | 3.5 | 2.5 | 1.5 |
| 铁(以Fe²⁺计,ppm)≤ | 50 | 30 | 30 | 10 |
| 灼烧残渣(%,/) | 0.2 | 0.2 | 0.02 | |
| 熔点(℃,/) | 102-105 | |||
| 乙醇溶解试验 | / | 合格 | 合格 | 合格 |
| 水溶解试验 | / | 合格 | 合格 | 合格 |
对甲苯磺酸为无色单斜片状或柱状晶体,易溶于乙醇和乙醚,稍溶于水和热苯。
用途对甲苯磺酸用作化学试剂、染料及有机合成的中间体。它还用于洗涤剂、塑料、涂料等方面,并广泛应用于医药(如强力霉素)、农药(如三氯杀螨醇)等领域。
生产方法 类别与特性上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对甲苯亚磺酸 p-toluene sulfinic acid 536-57-2 C7H8O2S 156.205 对甲苯磺酸甲酯 methyl p-toluene sulfonate 80-48-8 C8H10O3S 186.232 对甲苯磺酸乙酯 Ethyl tosylate 80-40-0 C9H12O3S 200.258 4-(三甲基硅烷基-甲基)-苯磺酸 4-(trimethylsilanyl-methyl)-benzenesulfonic acid 1899-92-9 C10H16O3SSi 244.387 3-甲基苯磺酸 m-toluenesulfonic acid 617-97-0 C7H8O3S 172.205 3,4-二甲基苯磺酸 3,4-dimethylbenzenesulfonic acid 618-01-9 C8H10O3S 186.232 对甲苯磺酸羟乙酯 2-hydroxyethyl 4-methylbenzenesulfonate 42772-85-0 C9H12O4S 216.258 对甲苯磺酸异丙酯 toluene-4-sulfonic acid isopropyl ester 2307-69-9 C10H14O3S 214.285 4-甲基苯磺酸丙酯 propyl tosylate 599-91-7 C10H14O3S 214.285 —— Ethyl p-toluenesulfinate 1858-86-2 C9H12O2S 184.259 对甲苯磺酸正丁酯 butyl para-toluenesulfonate 778-28-9 C11H16O3S 228.312 2-甲基苯磺酸 o-toluenesulfonic acid 88-20-0 C7H8O3S 172.205 3-氯-4-甲基苯磺酸 3-chloro-4-methylbenzenesulfonic acid 98-34-0 C7H7ClO3S 206.65 2,2-二甲基丙基4-甲基苯磺酸酯 neopentyl tosylate 2346-07-8 C12H18O3S 242.339 二乙二醇单对甲苯磺酸酯 5-tosyloxy-3-oxapentanol 118591-58-5 C11H16O5S 260.311 4-甲苯磺酸酐 p-toluenesulfonylanhydride 4124-41-8 C14H14O5S2 326.394 2-氰基苯甲酸 4-methylbenzenesulfonic acid n-hexylester 3839-35-8 C13H20O3S 256.366 —— n-butylethynyl tosylate 94957-45-6 C13H16O3S 252.334 —— 4-iodobenzenesulfonic acid 13035-63-7 C6H5IO3S 284.074 环丙基甲基4-甲基苯磺酸酯 cyclopropylmethyl tosylate 1015-45-8 C11H14O3S 226.296 —— toluene-4-sulfinic acid-anhydride 109042-83-3 C14H14O3S2 294.395 —— 2-p-toluenesulfonyloxy-1,3-propanediol 73684-56-7 C10H14O5S 246.284 4-甲基苯磺酸苯基甲基酯 benzyl tosylate 1024-41-5 C14H14O3S 262.329 —— cyclobutylmethyl 4-methylbenzenesulfonate 13295-53-9 C12H16O3S 240.323 —— benzaldehyde O-[(4-methylphenyl)sulphonyl]oxime 1517-28-8 C14H13NO3S 275.328 对甲磺酸苯乙酯 2-phenylethyl tosylate 4455-09-8 C15H16O3S 276.356 对甲苯亚磺酸苯酯 toluene-4-sulfonic acid phenyl ester 640-60-8 C13H12O3S 248.302 —— cyclopentylmethyl 4-methylbenzenesulfonate 21856-53-1 C13H18O3S 254.35 2-(4-甲基苯基)乙基4-甲基苯磺酸酯 2-(4-methylphenyl)ethyl tosylate 14503-40-3 C16H18O3S 290.383 —— 3-(toluene-4-sulfonyloxy)-propionic acid ethyl ester 34583-56-7 C12H16O5S 272.322 —— cyclopentyl tosylate 3558-06-3 C12H16O3S 240.323 环己基甲基4-甲基苯磺酸盐 cyclohexylmethyl p-toluenesulfonate 3725-11-9 C14H20O3S 268.377 —— cycloheptylmethyl 4-methylbenzenesulfonate 16472-98-3 C15H22O3S 282.404 —— 4-methylphenyl tosylate 3899-96-5 C14H14O3S 262.329 对甲苯磺酰胺 toluene-4-sulfonamide 70-55-3 C7H9NO2S 171.22 p-甲苯磺酸环己酯 cyclohexyl tosylate 953-91-3 C13H18O3S 254.35 4-甲基苯磺酰溴 toluene-p-sulfonyl bromide 1950-69-2 C7H7BrO2S 235.101 —— p-toluenesulfonyl iodide 1950-78-3 C7H7IO2S 282.102 对甲基苯磺酰氟 p-toluenesulfonyl fluoride 455-16-3 C7H7FO2S 174.196 —— toluene-4-sulfonic acid cyclooctyl ester 6597-09-7 C15H22O3S 282.404 - 1
- 2
- 3
- 4
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-hydroxymethylphenylsulfonic acid 122855-96-3 C7H8O4S 188.204 —— 4-(bromomethyl)benzenesulfonic acid 82835-61-8 C7H7BrO3S 251.101 4-甲酰基苯磺酸 benzaldehyde-4-sulfonic acid 5363-54-2 C7H6O4S 186.188 对甲苯磺酸甲酯 methyl p-toluene sulfonate 80-48-8 C8H10O3S 186.232 —— p-toluenesulfonic acid-d1 21013-41-2 C7H8O3S 173.197 对甲苯磺酸乙酯 Ethyl tosylate 80-40-0 C9H12O3S 200.258 —— vinyl 4-methylbenzenesulfonate 83813-73-4 C9H10O3S 198.243 氟甲醇4-甲基苯磺酸酯 fluoromethyl 4-methylbenzenesulfonate 114435-86-8 C8H9FO3S 204.222 3-甲基苯磺酸 m-toluenesulfonic acid 617-97-0 C7H8O3S 172.205 4-磺基苯甲酸酯 4-sulphobenzoic acid 636-78-2 C7H6O5S 202.188 对甲苯磺酸羟乙酯 2-hydroxyethyl 4-methylbenzenesulfonate 42772-85-0 C9H12O4S 216.258 对甲苯磺酸烯丙酯 allyl tosylate 4873-09-0 C10H12O3S 212.269 对甲苯磺酸异丙酯 toluene-4-sulfonic acid isopropyl ester 2307-69-9 C10H14O3S 214.285 —— difluoromethyl 4-methylbenzenesulfonate 14277-20-4 C8H8F2O3S 222.213 —— propynyl p-toluenesulfonate 94957-44-5 C10H10O3S 210.254 4-甲基苯磺酸丙酯 propyl tosylate 599-91-7 C10H14O3S 214.285 对甲苯磺酸氟乙酯 2-fluoroethyl tosylate 383-50-6 C9H11FO3S 218.249 2-溴乙基4-甲基苯磺酸盐 2-bromoethyl p-toluenesulfonate 19263-21-9 C9H11BrO3S 279.155 对甲苯磺酸2-甲氧基乙酯 2-methoxy-ethyl p-toluenesulfonyloxy ester 17178-10-8 C10H14O4S 230.285 1-甲基-4-[(4-甲基苯基)磺酰氧基甲氧基磺酰基]苯 methylene ditosylate 24124-59-2 C15H16O6S2 356.42 三甲基硅烷基对甲苯磺酸酯 4-Methyl-1-benzolsulfonsaeure-trimethylsilylester 17872-98-9 C10H16O3SSi 244.387 邻甲苯磺酸异丁酯 isobutyl p-toluenesulfonate 4873-56-7 C11H16O3S 228.312 对甲苯磺酸正丁酯 butyl para-toluenesulfonate 778-28-9 C11H16O3S 228.312 对甲苯磺酸 2-丁炔酯 toluene-4-sulfonic acid but-2-ynyl ester 56563-37-2 C11H12O3S 224.28 2,2-二氟乙基对甲苯磺酸盐 2,2-difluoroethyl 4-methylbenzenesulfonate 135206-84-7 C9H10F2O3S 236.239 2-甲基苯磺酸 o-toluenesulfonic acid 88-20-0 C7H8O3S 172.205 3-氯-4-甲基苯磺酸 3-chloro-4-methylbenzenesulfonic acid 98-34-0 C7H7ClO3S 206.65 —— 4-hydroxybutyl 4-methylbenzenesulfonate 6972-09-4 C11H16O4S 244.312 二乙二醇单对甲苯磺酸酯 5-tosyloxy-3-oxapentanol 118591-58-5 C11H16O5S 260.311 2,2-二甲基丙基4-甲基苯磺酸酯 neopentyl tosylate 2346-07-8 C12H18O3S 242.339 2-氨基甲苯-4-磺酸 3-amino-4-methylbenzenesulfonic acid 618-03-1 C7H9NO3S 187.219 二乙二醇双对甲苯磺酸酯 diethylene glycol ditosylate 7460-82-4 C18H22O7S2 414.5 —— 1,4-ditosyloxybutane 4724-56-5 C18H22O6S2 398.501 甲苯-4-磺酸戊酯 1-pentyl tosylate 4450-76-4 C12H18O3S 242.339 4-甲苯磺酸酐 p-toluenesulfonylanhydride 4124-41-8 C14H14O5S2 326.394 三乙二醇单对甲苯磺酸酯 8-tosyloxy-3,6-dioxaoctanol 77544-68-4 C13H20O6S 304.364 三乙二醇二(对甲苯磺酸酯) Triethylene glycol ditosylate 19249-03-7 C20H26O8S2 458.554 2-(2-甲氧基乙氧基)乙基4-甲基苯磺酸负离子 (2-methoxyethoxy)ethyl tosylate 50586-80-6 C12H18O5S 274.338 对甲苯磺酸三缩乙二醇单甲醚酯 2-(2-(2-methoxyethoxy)ethoxy)ethyl p-toluenesulfonate 62921-74-8 C14H22O6S 318.391 2,2,2-三氟乙基 对甲苯磺酸酯 2,2,2-Trifluoroethyl p-toluenesulfonate 433-06-7 C9H9F3O3S 254.23 四乙二醇单对甲苯磺酸酯 p-toluenesulfonic acid 2-[2-[2-(2-hydroxyethoxy)ethoxy]ethoxy]ethyl ester 77544-60-6 C15H24O7S 348.417 —— 2-(2-(2-ethoxyethoxy)ethoxy)ethyl 4-methylbenzenesulfonate 62921-75-9 C15H24O6S 332.418 —— toluene-4-sulfonic acid 2-[2-(2-{2-[2-(2-hydroxy-ethoxy)-ethoxy]-ethoxy}-ethoxy)-ethoxy]-ethyl ester 42749-28-0 C19H32O9S 436.524 非乙二醇单(对甲苯磺酰基)醚 2-[2-[2-[2-[2-[2-[2-[2-(2-hydroxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethyl 4-methylbenzenesulfonate 62573-11-9 C25H44O12S 568.683 六乙二醇单对甲苯磺酸酯 2,5,8,11,14,17-hexaoxanonadecan-19-yl 4-methylbenzenesulfonate 155887-96-0 C20H34O9S 450.551 对甲基苯磺酸乙酸酐 acetyl p-toluenesulfonate 26908-82-7 C9H10O4S 214.242 2-氰基苯甲酸 4-methylbenzenesulfonic acid n-hexylester 3839-35-8 C13H20O3S 256.366 —— 5-hydroxypentyl p-toluenesulfonate 95769-37-2 C12H18O4S 258.339 1,5-戊二醇双(对甲苯磺酸)酯 1,5-bis(p-toluenesulfonyloxy)pentane 24293-28-5 C19H24O6S2 412.528 2,4-己二炔-1,6-二基 双(4-甲基苯磺酸酯) 1,6-bis(p-toluenesulfonyloxy)-2,4-hexadiyne 32527-15-4 C20H18O6S2 418.491 —— (Z)-pent-3-enyl 4-methylbenzenesulfonate 781-05-5 C12H16O3S 240.323 —— (E)-pent-3-enyl 4-methylbenzenesulfonate 781-06-6 C12H16O3S 240.323 —— 4-chlorobutyl 4-methylbenzenesulfonate 20999-32-0 C11H15ClO3S 262.757 —— toluene-4-sulfonic acid pent-4-enyl ester 19300-54-0 C12H16O3S 240.323 2-二甲胺基乙基4-甲基苯磺酸 2-dimethylamino ethanol p-toluene sulfonate 123091-15-6 C11H17NO3S 243.327 戊-4-炔基对甲苯磺酸酯 4-pentynyl-1-tosylate 77758-50-0 C12H14O3S 238.307 —— 2-hydroxy-1-methylethyl 4-toluenesulfonate 54619-27-1 C10H14O4S 230.285 (R)-(-)-2-(对甲苯磺酸)-1,2-丙醇 R-2-(4-Methylbenzenesulfonyloxy)-1-propanol 69891-44-7 C10H14O4S 230.285 —— 2-(prop-2-yn-1-yloxy)ethyl 4-methylbenzenesulfonate 145916-41-2 C12H14O4S 254.307 —— n-butylethynyl tosylate 94957-45-6 C13H16O3S 252.334 —— 7-hydroxyheptyl 4-methylbenzenesulfonate 311805-13-7 C14H22O4S 286.392 对甲苯磺酸正辛酯 1-octyl p-toluenesulfonate 3386-35-4 C15H24O3S 284.42 —— 2-tosyloxybutane 50896-54-3 C11H16O3S 228.312 对甲苯磺酸十六烷基酯 tosylate de n-hexadecyle 6068-28-6 C23H40O3S 396.635 —— decyl p-toluenesulfonate 5509-08-0 C17H28O3S 312.474 十四烷基4-甲基苯磺酸盐 toluene-4-sulfonic acid tetradecyl ester 72422-53-8 C21H36O3S 368.581 4-甲基苯磺酸十八醇酯 n-octadecyl p-toluenesulfonate 3386-32-1 C25H44O3S 424.689 —— toluene-4-sulfonic acid 2-hydroxypropyl ester 32464-98-5 C10H14O4S 230.285 4-甲基苯磺酸 (R)-(-)-1-甲基丙酯 (R)-(-)-2-butyl tosylate 61530-30-1 C11H16O3S 228.312 —— 3,3-dimethyl-1-butynyl tosylate 90893-24-6 C13H16O3S 252.334 1,8-辛二醇,单(4-甲基苯磺酸酯) 8-hydroxyoctyl 4-methylbenzenesulfonate 103010-10-2 C15H24O4S 300.419 —— 2-oxopropyl tosylate 1666-19-9 C10H12O4S 228.269 对甲苯磺酸2-甲基丁酯 p-toluenesulfonic acid 2-methylbutyl ester 63526-71-6 C12H18O3S 242.339 —— 2-{[(4-methylphenyl)sulfonyl]oxy} ethyl acetate 19859-09-7 C11H14O5S 258.295 对甲苯磺酸三氟丙酯 3,3,3-trifluoropropyl-4-methylbenzene sulphonate 2342-67-8 C10H11F3O3S 268.257 甲苯-4-磺酸盐2-(环氧丙烷)乙酯 2-cyclopropylethyl 4-methylbenzenesulfonate 63064-31-3 C12H16O3S 240.323 —— 3,5-dichloro-4-methylbenzenesulphonic acid 2225-18-5 C7H6Cl2O3S 241.095 —— antimony tris(para-toluenesulphonate) —— C21H21O9S3Sb 635.34 4-甲基苯磺酸苯基甲基酯 benzyl tosylate 1024-41-5 C14H14O3S 262.329 (R,S)-1-对甲苯磺酰基甘油 (+/-)-2,3-dihydroxypropyl tosylate 73073-07-1 C10H14O5S 246.284 —— phenylethynyl tosylate 94957-46-7 C15H12O3S 272.324 2,2双(甲苯磺酰氧基甲基)丙烷-1,3二基双(4甲基苯磺酸酯) pentaerythritol tetratosylate 1522-89-0 C33H36O12S4 752.906 —— (E)-styryl 4-methylbenzenesulfonate —— C15H14O3S 274.34 十八碳-9-烯-1-醇4-甲基苯磺酸酯 oleyl p-toluenesulfonate 6110-54-9 C25H42O3S 422.673 对甲磺酸苯乙酯 2-phenylethyl tosylate 4455-09-8 C15H16O3S 276.356 —— 3-cyclohexyl-1-(p-toluenesulfonyloxy)propane 51584-56-6 C16H24O3S 296.431 —— 1-(tosyloxy)-2-butanone 80520-04-3 C11H14O4S 242.296 —— (R,S)-2-hydroxy-3-chloropropyl p-toluenesulfonate 102129-99-7 C10H13ClO4S 264.73 —— (R)-3-chloro-2-hydroxypropyl 4-methylbenzenesulfonate 83398-53-2 C10H13ClO4S 264.73 —— (S)-3-chloro-2-hydroxypropyl-1-(toluene-4-sulfonate) 67800-61-7 C10H13ClO4S 264.73 4-甲基苯-1,3-二磺酸 toluene-2,4-disulfonic acid 121-04-0 C7H8O6S2 252.269 甲苯磺酸-(2-羟基-3-丁烯)酯 2-hydroxybut-3-enyl-4-methylbenzenesulfonate 138332-13-5 C11H14O4S 242.296 对甲苯亚磺酸苯酯 toluene-4-sulfonic acid phenyl ester 640-60-8 C13H12O3S 248.302 —— 4-aminobutan-2-ol tosylate —— C11H17NO3S 243.327 —— 3-fluoro-1,2-propanediol 1-O-(4-methylbenzene)sulphonate 142545-51-5 C10H13FO4S 248.275 —— cinnamyl tosylate 19627-30-6 C16H16O3S 288.367 —— 6-azidohexyl 4-methylbenzenesulfonate —— C13H19N3O3S 297.378 —— (5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenyl-4-methylbenzenesulfonate 56401-77-5 C27H40O3S 444.679 西那卡塞杂质105 3-phenylpropyl 4-methylbenzenesulfonate 3742-75-4 C16H18O3S 290.383 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
反应信息
-
作为反应物:参考文献:名称:在钛-Salan 催化剂上用 H2O2 催化对映选择性氧化大块烷基芳基硫醚摘要:报道了一种用过氧化氢以良好至高产率和对映选择性(高达 98.5% ee)氧化大体积(优选芳基苄基取代)硫醚的简单有效的催化程序。在串联立体会聚对映选择性氧化和动力学拆分过程中实现了高光学产率。通过改变浓度和温度可以找到亚砜产率和对映选择性之间的合理平衡。报道了用于制备更具立体选择性的催化剂的钛-salan 催化剂的改进合成。DOI:10.1002/ejoc.201100557
-
作为产物:参考文献:名称:Removal of alkyl alkanesulfonate esters from alkanesulfonic acids and other organic media摘要:提供了从水性或无水性组合物中去除烷基烷磺酸酯的方法。该发明提供了将化学式为RSO3R′的烷基烷磺酸酯转化为化学式为RSO3H的相应酸的方法。烷基烷磺酸酯存在于有机介质中,该介质可能含有大量水,也可能是无水或基本无水的。在某些实施例中,该发明提供了通过去除烷基烷磺酸酯来净化水性或无水烷磺酸的方法。公开号:US20060030725A1
-
作为试剂:参考文献:名称:基于TMV-CP的抗植物病毒α-酰胺磷酸衍生物的合理设计与发现摘要:烟草花叶病毒外壳蛋白(TMV-CP)对于病毒的复制、运动和传播以及宿主植物的免疫系统识别它是不可或缺的。它构成病毒颗粒的最外层,是病毒结构的重要组成部分。 TMV-CP 对于启动和延长病毒组装至关重要,在烟草花叶病毒 (TMV) 的自组装过程中发挥着至关重要的作用。该研究采用 TMV-CP 作为虚拟筛选的主要目标,从中获取了包含 43,417 种化合物的库,并被选为先导化合物。因此,设计并合成了一系列α-酰胺磷酸衍生物,表现出显着的抗TMV功效。发现合成的化合物有利于治疗 TMV,其疗效略优于宁南霉素 (NNM) (EC = 304.54 µg/mL),EC 为 291.9 µg/mL。此外,其灭活活性 (EC = 63.2 µg/mL) 与 NNM (EC = 67.5 µg/mL) 相当,且具有与 NNM (EC = 219.7 µg/mL) 类似的保护活性 (EC = 228.9 µg/mL)。微量热分析表明DOI:10.1016/j.bioorg.2024.107415
文献信息
-
[EN] BENZAMIDE OR BENZAMINE COMPOUNDS USEFUL AS ANTICANCER AGENTS FOR THE TREATMENT OF HUMAN CANCERS<br/>[FR] COMPOSÉS BENZAMIDE OU BENZAMINE À UTILISER EN TANT QU'ANTICANCÉREUX POUR LE TRAITEMENT DE CANCERS HUMAINS申请人:UNIV TEXAS公开号:WO2017007634A1公开(公告)日:2017-01-12The described invention provides small molecule anti-cancer compounds for treating tumors that respond to cholesterol biosynthesis inhibition. The compounds selectively inhibit the cholesterol biosynthetic pathway in tumor-derived cancer cells, but do not affect normally dividing cells.
-
BENZOTHIOPHENE INHIBITORS OF RHO KINASE申请人:Kahraman Mehmet公开号:US20080021026A1公开(公告)日:2008-01-24The present invention relates to compounds and methods which may be useful as inhibitors of Rho kinase for the treatment or prevention of disease.本发明涉及化合物和方法,这些化合物和方法可能作为Rho激酶的抑制剂在治疗或预防疾病方面有用。
-
SYNTHESIS OF CYCLIC AMIDINES申请人:Lentzen George公开号:US20110178292A1公开(公告)日:2011-07-21The invention relates to an innovative method for synthesis of cyclic amidines. The synthesis starts from a β-, γ- or δ-lactone which is twofold brominated. After esterification of the carboxyl function, the bromine atoms are nucleophilically substituted and the corresponding diamino compound is obtained. The ring closure to the cyclic amidine is accomplished subsequently by reaction with orthoester, imidate or thioimidate. Owing to interposing additional steps for recovery of the diamino compound in enantiomerically pure form, the enantiomers of the cyclic amidines can be stereoselectively synthesized.
-
N-type calcium channel blockers申请人:Pajouhesh Hassan公开号:US20050165065A1公开(公告)日:2005-07-28The invention relates to novel 3-amino pyrrolidine derivatives, as well as methods for modulating calcium channel activity and for treating conditions associated with calcium channel function. In particular, the compounds generally contain at least one benzhydril moiety, and are useful in treating conditions which benefit from blocking calcium ion channels.
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
表征谱图
-
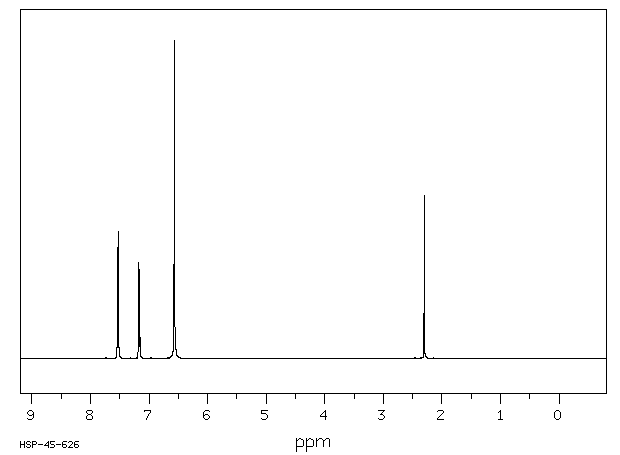
氢谱1HNMR
-
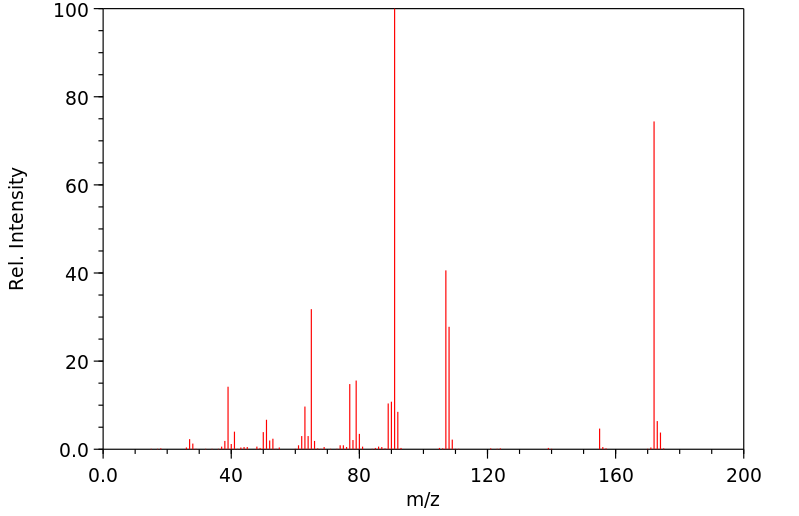
质谱MS
-
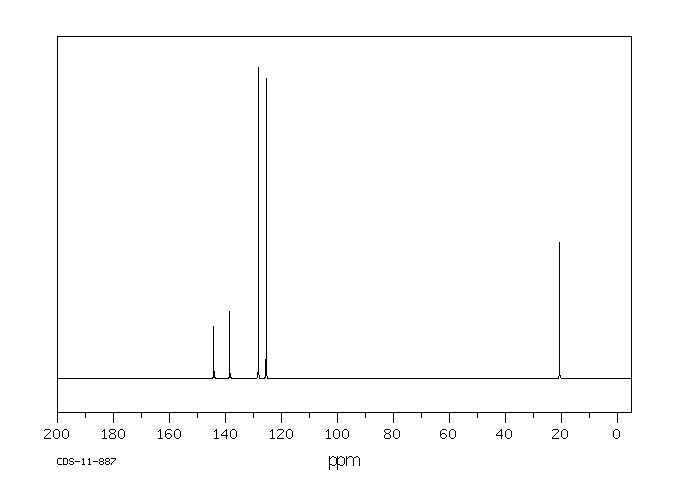
碳谱13CNMR
-
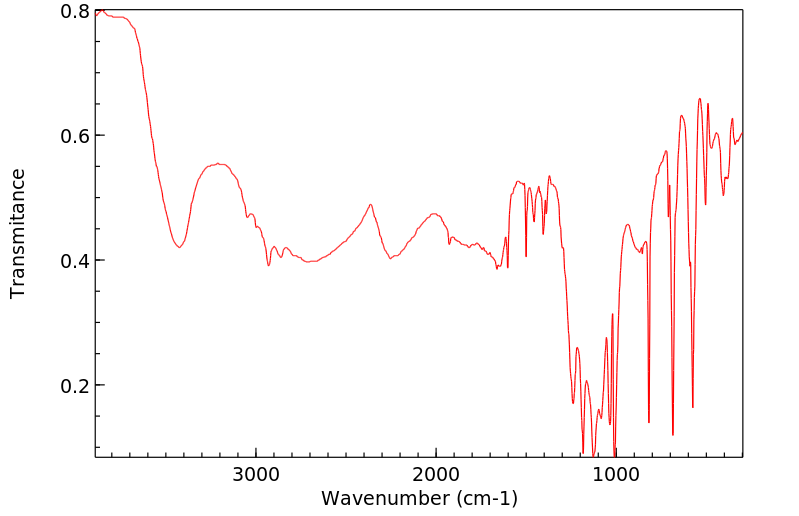
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫