代谢
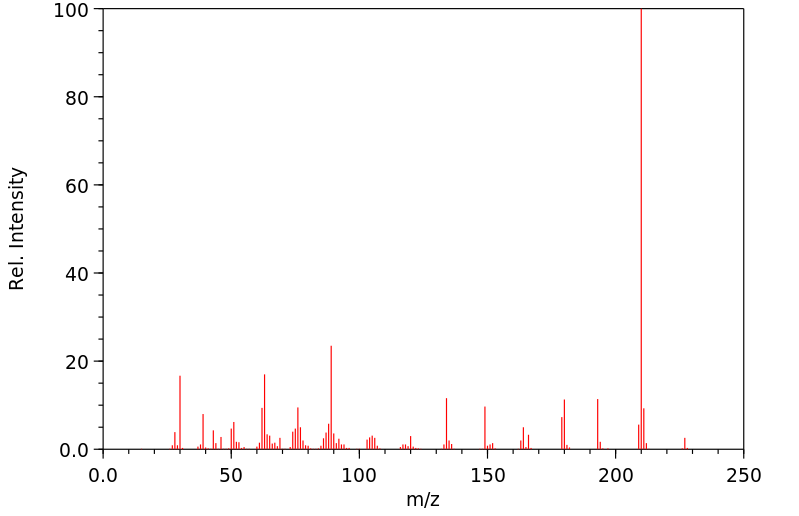
2,4,6-三硝基甲苯(TNT)在大鼠、小鼠、兔和狗体内的代谢进行了研究,通过口服、皮肤或气管内给药单一剂量的(14)C-环标记化合物。在所有物种中,TNT都被广泛代谢;放射性主要通过尿液以葡萄糖苷酸结合物的形式排出。大多数代谢物是还原产物,包括2-和4-羟基胺以及2-和4-单氨基二硝基和2,6-和4,6-二氨基一硝基衍生物。偶尔会检测到微量的TNT、三硝基苯甲醇和三硝基苯甲酸。
The metabolism of 2,4,6-trinitrotoluene (TNT) was studied in rats, mice, rabbits, and dogs following oral, dermal, or intratracheal admin of single doses of (14)C-ring labeled compound. TNT was extensively metabolized in all species; radioactivity was excreted in urine primarily as the glucuronide conjugates. Most metabolites were reduction products including the 2- and 4-hydroxylamine and 2- and 4-monoaminodinitro and 2,6- and 4,6-diaminomononitro derivatives. Trace quantities of TNT, trinitrobenzyl alcohol, and trinitrobenzoic acid were detected occasionally.
来源:Hazardous Substances Data Bank (HSDB)