3,5-二硝基苯胺 | 618-87-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:160-162 °C(lit.)
-
沸点:316.77°C (rough estimate)
-
密度:1.6010
-
保留指数:321.42
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:13
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:118
-
氢给体数:1
-
氢受体数:5
安全信息
-
危险等级:6.1(a)
-
危险品标志:T
-
安全说明:S28,S37,S45
-
危险类别码:R23/24/25,R33
-
WGK Germany:2
-
RTECS号:BX9200100
-
海关编码:2921420090
-
包装等级:II
-
危险类别:6.1(a)
-
危险品运输编号:UN 1596 6.1/PG 2
-
储存条件:储存注意事项: - 储存于阴凉、通风的库房。 - 远离火种、热源,包装密封。 - 应与氧化剂、酸类及食用化学品分开存放,切忌混储。 - 配备相应品种和数量的消防器材。 - 储区应备有合适的材料收容泄漏物。
SDS
| 国标编号: | 61778 |
| CAS: | 618-87-1 |
| 中文名称: | 3,5-二硝基苯胺 |
| 英文名称: | 3,5-dinitroaniline |
| 别 名: | |
| 分子式: | C 6 H 5 N 3 O 4 ;(O 2 N) 2 C 6 H 3 NH 2 |
| 分子量: | 183.13 |
| 熔 点: | 159~160℃ |
| 密 度: | |
| 蒸汽压: | |
| 溶解性: | 微溶于苯,溶于乙醇、乙醚 |
| 稳定性: | 稳定 |
| 外观与性状: | 黄色针状结晶 |
| 危险标记: | 14(毒害品) |
| 用 途: | 用于有机合成 |
2.对环境的影响: 一、健康危害 侵入途径:吸入、食入、经皮吸收。 健康危害:对眼睛、粘膜、呼吸道及皮肤有刺激作用。吸收进入体内导致形成高铁血红蛋白而引起紫绀。吸入、摄入或经皮肤吸收可能致死。 二、毒理学资料及环境行为 危险特性:遇明火、高热可燃。与强氧化剂可发生反应。急剧加热时可发生爆炸。 燃烧(分解)产物:一氧化碳、二氧化碳、氧化氮。 3.现场应急监测方法: 4.实验室监测方法: 光度法测定,波长400nm,俄国刊物,水.aHaЛ.XHM.1990,45(4),815~817 《分析化学文摘 》1992-1992 5.环境标准: 6.应急处理处置方法: 一、泄漏应急处理 隔离泄漏污染区,周围设警告标志,建议应急处理人员戴好防毒面具,穿化学防护服。不要直接接触泄漏物,用清洁的铲子收集于干燥净洁有盖的容器中,运至废物处理场所或用沙土混合逐渐倒入稀盐酸中(1体积浓盐酸加2体积水稀释),放置24小时,然后废弃。如大量泄漏,收集回收或无害处理后废弃。 二、防护措施 呼吸系统防护:空气中浓度较高时,佩带防毒面具。紧急事态抢救或逃生时,应该佩戴自给式呼吸器。 眼睛防护:戴化学安全防护眼镜。 防护服:穿紧袖工作服,长统胶鞋。 手防护:戴橡皮手套。 其它:工作现场禁止吸烟、进食和饮水。及时换洗工作服。工作前不饮酒,用温水洗澡。进行就业前和定期的体检。 三、急救措施 皮肤接触:立即脱去污染的衣着,用肥皂水及清水彻底冲洗。注意手、足和指甲等部位。 眼睛接触:立即提起眼睑,用大量流动清水或生理盐水冲洗。 吸入:迅速脱离现场至空气新鲜处。呼吸困难时给输氧。呼吸停止时,立即进行人工呼吸。就医。 食入:误服者给漱口,饮水,洗胃后口服活性炭,再给以导泻。就医。 灭火方法:雾状水、二氧化碳、砂土、干粉、泡沫。
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,3,5-三硝基苯 TNB 99-35-4 C6H3N3O6 213.106 —— N-Hydroxy-3,5-dinitroaniline 88106-09-6 C6H5N3O5 199.123 3,5-二异氰酸硝基苯 1-isocyanato-3,5-dinitrobenzene 59776-60-2 C7H3N3O5 209.118 1-氟-2,4-二硝基苯 1-fluoro-2,4-dinitrobenzene 369-18-6 C6H3FN2O4 186.099 3,5-二硝基溴苯 3,5-dinitrobromobenzene 18242-39-2 C6H3BrN2O4 247.005 2,4,6-三硝基甲苯 2,4,6-Trinitrotoluene 118-96-7 C7H5N3O6 227.133 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,3,5-三硝基苯 TNB 99-35-4 C6H3N3O6 213.106 1,3-二硝基苯 1,3-Dinitrobenzene 99-65-0 C6H4N2O4 168.109 3,5-二氨基硝基苯 5-nitrobenzene-1,3-diamine 5042-55-7 C6H7N3O2 153.14 —— 3,5-dinitro-N-methylaniline 70872-16-1 C7H7N3O4 197.15 3,5-二异氰酸硝基苯 1-isocyanato-3,5-dinitrobenzene 59776-60-2 C7H3N3O5 209.118 N,N -二甲基-3,5-硝基苯 N,N-dimethyl-3,5-dinitroaniline 46429-76-9 C8H9N3O4 211.177 —— formic acid-(3,5-dinitro-anilide) 54338-42-0 C7H5N3O5 211.134 1-氯-3,5-二硝基苯 1-chloro-3,5-dinitrobenzene 618-86-0 C6H3ClN2O4 202.554 1-碘基-3,5-二硝基苯 3,5-dinitroiodobenzene 6276-04-6 C6H3IN2O4 294.005 3,5-二硝基溴苯 3,5-dinitrobromobenzene 18242-39-2 C6H3BrN2O4 247.005 3,5-二硝基酚 3,5-dinitrophenol 586-11-8 C6H4N2O5 184.108 —— 2-amino-3,5-dinitrophenylamine 3694-51-7 C6H6N4O4 198.138 3-氯-5-硝基苯胺 3-chloro-5-nitroaniline 5344-44-5 C6H5ClN2O2 172.571 1,3,5-三氨基-2,4,6-三硝基苯 2,4,6-triamino-1,3,5-trinitrobenzene 3058-38-6 C6H6N6O6 258.15 —— N-(3,5-dinitrophenyl)-acetamide 38802-18-5 C8H7N3O5 225.161 —— 3,5-Dinitro-thioanisol 41085-57-8 C7H6N2O4S 214.202 —— 3-amino-5-nitroacetanilide 42380-21-2 C8H9N3O3 195.178 —— N,N'-bis-(3,5-dinitro-phenyl)-urea —— C13H8N6O9 392.241 —— 2-bromo-3,5-dinitro-aniline 116529-41-0 C6H4BrN3O4 262.019 1,3-二(乙酰氨基)-5-硝基苯 N-(3-acetylamino-5-nitrophenyl)acetamide 17178-95-9 C10H11N3O4 237.215 戊硝基苯胺 2,3,4,5,6-pentanitroaniline 1,2-dichloroethane 21985-87-5 C6H2N6O10 318.116 1,3-二硝基-5-苯基苯 3,5-dinitro-1,1′-biphenyl 56813-80-0 C12H8N2O4 244.207 —— 3',5'-Dinitro-isobutyranilid 54338-34-0 C10H11N3O5 253.214 烯丙基-3,5-二硝基苯 allyl-3,5-dinitrobenzene 250782-02-6 C9H8N2O4 208.174 —— 3,5-dinitrobenzeneboronic acid —— C6H5BN2O6 211.926 —— 2,2-difluoro-N-(3,5-dinitrophenyl)acetamide 330628-61-0 C8H5F2N3O5 261.142 —— (3,5-dinitro-phenyl)-carbamic acid ethyl ester 50571-36-3 C9H9N3O6 255.187 —— N-(3,5-dinitrophenyl)-2,2-dimethylpropionamide 367970-06-7 C11H13N3O5 267.241 —— N-methyl-m,m-dinitroacetanilide 54338-36-2 C9H9N3O5 239.188 - 1
- 2
- 3
反应信息
-
作为反应物:参考文献:名称:Synthesis of polynitrodiazophenols摘要:DOI:10.1021/jo00363a032
-
作为产物:描述:参考文献:名称:用水合肼选择性还原 1,3,5-三硝基苯中的一个、两个或三个硝基摘要:开发了一种在氯化铁和木炭存在下使用水合肼连续选择性还原 1,3,5-三硝基苯中的一个、两个或三个硝基的方法。该方法提供了一种从 1,3,5-三硝基苯一锅合成 3,5-二硝基苯胺、1,3-二氨基-5-硝基苯或 1,3,5-三氨基苯的方法。DOI:10.1007/s11172-006-0356-2
文献信息
-
Efficient one-pot transformation of aminoarenes to haloarenes using halodimethylisulfonium halides generated in situ作者:Woonphil Baik、Wanqiang Luan、Hyun Joo Lee、Cheol Hun Yoon、Sangho Koo、Byeong Hyo KimDOI:10.1139/v05-026日期:2005.3.1
Halodimethylsulfonium halide 1, which is readily formed in situ from hydrohaloic acid and DMSO, is a good nucleophilic halide. This activated nucleophilic halide rapidly converts aryldiazonium salt prepared in situ by the same hydrohaloic acid and nitrite ion to aryl chlorides, bromides, or iodides in good yield. The combined action of nitrite ion and hydrohaloic acid in DMSO is required for the direct transformation of aromatic amines, which results in the production of aryl halides within 1 h. Substituted compounds with electron-donating or -withdrawing groups or sterically hindered aromatic amines are also smoothly transformed to the corresponding aromatic halides. The only observed by-product is the deaminated arene (usually <7%). The isolated aryldiazonium salts can also be converted to the corresponding aryl halides using 1. The present method offers a facile, one-step procedure for transforming aminoarenes to haloarenes and lacks the environmental pollutants that usually accompany the Sandmeyer reaction using copper halides. Key words: aminoarenes, haloarenes, halodimethylsulfonium halide, halogenation, amination.
卤二甲基亚砜卤化物1是一种良好的亲核卤化物,可在现场由氢卤酸和二甲亚砜形成。这种活化的亲核卤化物迅速将由相同的氢卤酸和亚硝酸根在现场制备的芳基重氮盐转化为芳基氯化物、溴化物或碘化物,收率较高。在DMSO中,亚硝酸根和氢卤酸的联合作用是直接转化芳香胺的必要条件,从而在1小时内产生芳基卤化物。带有电子给体或吸引基团或有立体位阻的芳香胺的取代化合物也可顺利转化为相应的芳香卤化物。观察到的唯一副产物是去氨基芳烃(通常<7%)。孤立的芳基重氮盐也可以使用1转化为相应的芳基卤化物。该方法提供了一种简便的、一步法的程序,用于将氨基芳烃转化为卤代芳烃,并且不伴随通常伴随使用铜卤化物进行桑迈尔反应的环境污染物。关键词:氨基芳烃,卤代芳烃,卤二甲基亚砜卤化物,卤化,胺化。 -
Sandmeyer reactions. Part 7.1 An investigation into the reduction steps of Sandmeyer hydroxylation and chlorination reactions作者:Peter Hanson、Jason R. Jones、Alec B. Taylor、Paul H. Walton、Allan W. TimmsDOI:10.1039/b200748g日期:2002.5.22aqueous solution, the reduction steps have been investigated by means of correlation analyses of the effects of diazonium ion substitution on the rates of reduction. For simple hydroxylation, a change of behaviour between diazonium ions substituted by electron donor groups and those substituted by electron acceptor groups is interpreted as a change within an inner-sphere process from rate-determining electron对于桑德迈尔水溶液中的羟基化和氯化反应,通过相关分析重氮离子取代对还原速率的影响,研究了还原步骤。对于简单的羟基化,被电子给体基团取代的重氮离子与被电子受体基团取代的重氮离子之间的行为变化被解释为内球过程中从速率确定电子转移到反应物速率确定缔合的变化。相比之下,对于柠檬酸盐促进的羟基化,类似的行为变化可解释为内层和外层电子转移之间的变化。对于氯化作用,在所检查的取代基范围内没有机理上的变化,但其行为方式与内球机理一致。根据重氮离子取代和催化剂连接对各种反应性氧化还原对的还原电势和自交换速率的影响,合理化了各种行为方式。重氮离子的还原反应和其他亲电反应的比较相关性分析用于支持关于Sandmeyer还原步骤的高级论证。建议铜我还原剂反应经由亲核桥连配体的重氮Ñ β以得到瞬变ž-adducts其为前体配合物和活化用于电子转移包括关于N-N键的旋转。
-
Fluorinated biphenyls from aromatic arylations with pentafluorobenzenediazonium and related cations. Competition between arylation and azo coupling作者:Dmitry Kosynkin、T. Michael Bockman、Jay K. KochiDOI:10.1039/a701745f日期:——High yields of the mixed perfluorinated biaryls (C6F5–Ar) are obtained by the catalytic dediazoniation of the pentafluorobenzenediazonium salt (C6F5N2+BF4–) in acetonitrile solutions containing various aromatic substrates (ArH) together with small amounts of iodide salts. Activated (electron-rich) as well as deactivated (electron-poor) arenes are successfully pentafluorophenylated by this method. The arylation is distinct from the azo coupling of the same substrates, which takes place in the absence of the iodide catalyst and yields the corresponding diazene (C6F5NN–Ar) as product. The catalytic role of iodide, and the isomeric product distributions obtained with this procedure indicate that the arylation proceeds via the pentafluorophenyl radical in a efficient homolytic chain process. Since azo coupling involves electrophilic aromatic substitution of electron-rich ArH by C6F5N2+, the two competing pathways are distinct and do not have reactive intermediates in common.通过催化脱重氮化反应,在含有各种芳香族底物(ArH)及少量碘化盐的乙腈溶液中,五氟苯重氮盐( N2+BF4–)能够高效生成混合全氟联苯(C6F5–Ar),产率较高。无论是活化的(电子富集的)还是去活化的(电子贫乏的)芳香族化合物,都能通过此方法成功实现五氟苯基化。这种芳基化反应与同种底物的偶氮耦合反应截然不同,后者在无碘催化剂存在下进行,产物为相应重氮烯( NN–Ar)。碘化物在催化中的作用以及由此过程获得的不同异构产物分布表明,芳基化反应是通过高效的均裂链式过程,经由五氟苯基自由基进行的。由于偶氮耦合涉及电子富集的ArH通过亲电芳香取代反应与 N2+的结合,这两种竞争路径是不同的,且没有共同的反应中间体。
-
ω-Phthalimidoalkyl Aryl Ureas as Potent and Selective Inhibitors of Cholesterol Esterase作者:Florian M. Dato、Miriam Sheikh、Rocky Z. Uhl、Alexandra W. Schüller、Michaela Steinkrüger、Peter Koch、Jörg-Martin Neudörfl、Michael Gütschow、Bernd Goldfuss、Markus PietschDOI:10.1002/cmdc.201800388日期:2018.9.6with an attached 3,5‐bis(trifluoromethyl)phenyl group and the aromatic character of the ω‐phthalimide residue were most important for inhibitory activity. In addition, an alkyl chain composed of three or four methylene groups, connecting the urea and phthalimide moieties, was found to be an optimal spacer for inhibitors. The so‐optimized compounds 2 [1‐(3,5‐bis(trifluoromethyl)phenyl)‐3‐(3‐(1,3‐di胆固醇酯酶(CEase)是一种丝氨酸水解酶,被认为与动脉粥样硬化有关,因此与冠心病有关,被认为是抑制剂发展的目标。在一个小的ω-邻苯二甲酰亚胺基烷基芳基尿素文库的结构-活性关系研究中,我们用新的荧光测定法研究了重组人和鼠类CEase。带有3,5-双(三氟甲基)苯基的尿素基序和ω-邻苯二甲酰亚胺残基的芳香特性对于抑制活性最重要。另外,发现由三个或四个连接尿素和邻苯二甲酰亚胺部分的亚甲基组成的烷基链是抑制剂的最佳间隔基。如此优化的化合物2 [1-(3,5-双(三氟甲基)苯基)-3-(3-(1,3-二氧代异吲哚啉-2-基)丙基)脲]和21[1-(3,5-双(三氟甲基)苯基)-3-(4-(1,3- dioxoisoindolin -2-基)丁基)脲]显示解离常数(ķ我的1-19μ)米上的两种CEase并显示出竞争性抑制作用(对人类酶而言为2种,对鼠类酶而言为21种)或非竞争性抑制方式。ω-邻苯二甲
-
Acidic ionic liquid supported on silica-coated magnetite nanoparticles as a green catalyst for one-pot diazotization–halogenation of the aromatic amines作者:Jalal IsaadDOI:10.1039/c4ra05705h日期:——
Acidic ionic liquid was immobilized on silica-coated magnetite nanoparticles (Fe3O4@SILnP) and used as an efficient heterogeneous catalyst for the diazotization–iodination reaction of different aromatic amines under solvent-free conditions at room temperature.
表征谱图
-
氢谱1HNMR
-
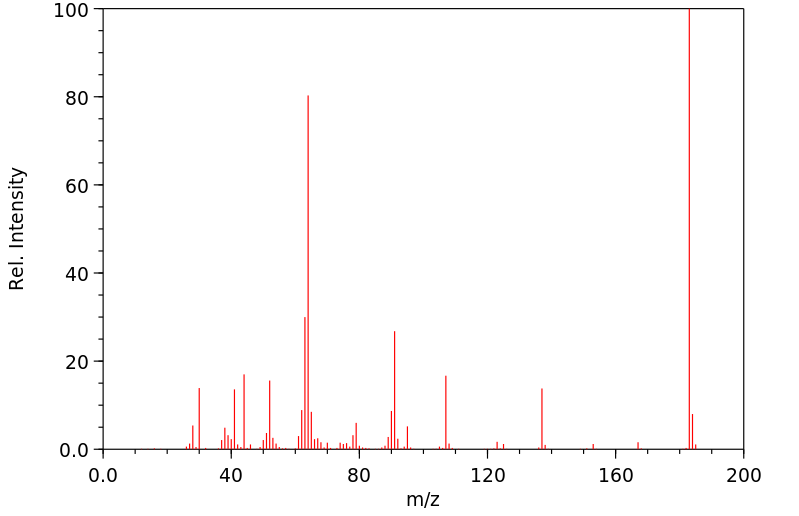
质谱MS
-
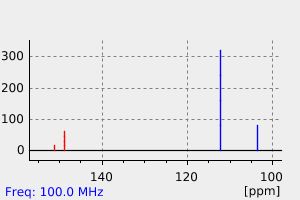
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息