2-己酮 | 591-78-6
中文名称
2-己酮
中文别名
丁基甲基酮;甲丁酮;2-已酮;甲基丁基甲酮;甲基正丁酮;MBK;甲基丁基(甲)酮;甲基正丁基甲酮;己酮-2;甲基正丁基酮
英文名称
2-Hexanone
英文别名
n-hexan-2-one;hexan-2-one;hexanone;trans-2-hexanal
CAS
591-78-6
化学式
C6H12O
mdl
MFCD00009482
分子量
100.161
InChiKey
QQZOPKMRPOGIEB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-57 °C
-
沸点:127 °C(lit.)
-
密度:0.812 g/mL at 25 °C(lit.)
-
闪点:95 °F
-
溶解度:溶于丙酮、乙醇和乙醚 (Weast, 1986)
-
介电常数:14.6(15℃)
-
暴露限值:NIOSH REL: TWA 1 ppm (4 mg/m3), IDLH 1,600 ppm; OSHA PEL: TWA 100 ppm (410 mg/m3); ACGIH TLV: TWA 5 ppm, STEL 10 ppm (adopted).
-
LogP:1.380
-
物理描述:Methyl butyl ketone appears as a clear colorless liquid. Flash point 95°F. Less dense than water. Vapors heavier than air.
-
颜色/状态:Colorless liquid
-
气味:Characteristic acetone like odor, but more pungent
-
蒸汽密度:3.45 (NTP, 1992) (Relative to Air)
-
蒸汽压力:11.6 mm Hg at 25 °C
-
大气OH速率常数:9.10e-12 cm3/molecule*sec
-
自燃温度:795 °F (423 °C)
-
粘度:0.62 cP at 20 °C
-
燃烧热:-16,000 Btu/lb = -8,940 cal/g = -374X10+5 J/kg
-
汽化热:36.05 kJ/mol
-
表面张力:25.4 dyn/cm at 20 °C
-
电离电位:9.34 eV
-
气味阈值:ODOR 3 PPM: 3= EASILY NOTICEABLE; IRRITATION 2 PPM: 2= FAINT.
-
折光率:Index of refraction: 1.4024 at 20 °C/D
-
相对蒸发率:1.0 (n-butyl acetate = 1)
-
保留指数:760.6;771;770;760;763;767.9;765.86;766.66;766.82;767.07;767.68;768.37;768.7;769.32;769.68;771.05;772.24;774.21;772;765.83;767.41;769.69;770.6;770.63;766.8;766.9;766.97;767.1;767.3;768.04;769;767.7;767;767;768;769;774;774;775;777;761;770;766.6;772;770;771;768;775;773;778;768;768;769;769;747;768;768;769;771;771;769;756;767;772;767;769.7;768;767;761;768;764.3;769.5;771;770;768;761;768;765;768;759;761;761;761;771;787;747;772;769;769;775;766.9;767;760;787;779;751
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:7
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.833
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
ADMET
代谢
调查人员研究了豚鼠对MnBK的生物转化,并得出结论,主要的代谢物是己二酮。
/Investigators/ studied the biotransformation of MnBK by the guinea pig and concluded that the primary metabolite was hexanedione.
来源:Hazardous Substances Data Bank (HSDB)
代谢
/2-Hexanone/ is metabolized by the liver to a common toxic metabolite, 2,5-hexanedione. This compound is a direct neurotoxin.
来源:Hazardous Substances Data Bank (HSDB)
代谢
In guinea pigs and rats, methyl butyl ketone was oxidatively and reductively metabolized. 2,5-Dimethyl-2,3-dihydrofuran; 5-hydroxy-2-hexanone; 2-hexanol; and 2,5-hexanedione were compounds identified in serum of guinea pigs dosed with MBK.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Charles River大鼠的尿代谢物:2-己醇;5-羟基-2-己酮;2,5-己二酮;2,5-二甲基呋喃;γ-戊内酯;正亮氨酸;尿素。显然,α-氧化成二氧化碳是一种解毒机制,而ω-1氧化会导致活化。
Urinary metabolites from Charles river rats: 2-hexanol; 5-hydroxy-2-hexanone; 2,5-hexanedione; 2,5-dimethylfuran; gamma-valerolactone; norleucine; and urea. Apparently, alpha-oxidation to carbon dioxide is detoxification mechanism and omega-1 oxidation leads to activation.
来源:Hazardous Substances Data Bank (HSDB)
代谢
己酮-2通过摄入、吸入和皮肤途径被吸收,然后广泛分布在整个身体中,肝脏和血液中的含量最高。其代谢过程可能与其他脂肪酮类似,通过还原成相应的二级醇,即己醇-2。另一种途径是氧化成相应的醇,5-羟基-2-己酮,然后进一步氧化成二酮2,5-己二酮。己酮-2及其代谢物通过呼出或尿液排出体外。(L176)
2-Hexanone is absorbed via ingestion, inhalation, and dermal routes, then distributed widely throughout the body, with the highest levels in the liver and blood. Metabolism is likely similar to that of other aliphatic ketones, proceeding via reduction to the corresponding secondary alcohol, 2-hexanol. An alternate pathway is oxidation to the corresponding alcohol, 5-hydroxy-2-hexanone, followed by further oxidation to the diketone 2,5-hexanedione. 2-Hexanone and its metabolites are excreted via exhalation or in the urine. (L176)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
己酮-2的毒性被认为是由于其代谢物,特别是己二酮-2,5,与神经组织的轴突成分发生共价结合,并抑制与该组织产生能量相关的酶。己酮-2和己二酮-2,5还可能抑制依赖于巯基的酶,如果糖-6-磷酸激酶和甘油醛-3-磷酸脱氢酶,以及某些肌酸激酶和腺苷酸激酶,损害能量代谢,从而导致轴突退化。此外,己二酮-2,5能够将神经丝共价交联,导致大的轴突肿胀。
2-Hexanone's toxicity is believed to be caused by covalent binding of its metabolites, especially 2,5-hexanedione, with axonal components of nerve tissue and inhibition of enzymes associated with the production of energy in this tissue. 2-Hexanone and 2,5-hexanedione may also inhibit sulfhydryl-dependent enzymes such as fructose-6-phosphate kinase and glyceraldehyde-3-phosphate dehydrogenase, as well as certain creatine kinases and adenylate kinases, impairing energy metabolism and subsequently resulting in axon deterioration. In addition, 2,5-hexanedione can covalently cross-links neurofilaments, causing large axonal swellings. (L176, A124, A125)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
对人类无致癌性(未列入国际癌症研究机构IARC清单)。
No indication of carcinogenicity to humans (not listed by IARC).
来源:Toxin and Toxin Target Database (T3DB)
毒理性
吸入2-己酮会影响神经和生殖系统。这可能包括诸如周围神经病和发育缺陷等病理状况。
Breathing 2-hexanone affects the nervous and reproductive systems. This may include pathologies such as peripheral neuropathy and developmental defects. (L176)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
该物质可以通过吸入和透过皮肤被身体吸收。
The substance can be absorbed into the body by inhalation and through the skin.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
吸入,皮肤吸收,吞食,皮肤和/或眼睛接触
inhalation, skin absorption, ingestion, skin and/or eye contact
来源:The National Institute for Occupational Safety and Health (NIOSH)
吸收、分配和排泄
吸入是主要的接触途径;然而,皮肤接触可能导致皮肤刺激,这种吸收可能对慢性接触和多发性神经病有所贡献。摄入的报告非常罕见。
Inhalation is the primary route of exposure; however, dermatologic exposure can lead to skin irritation and this absorption may contribute to chronic exposure and polyneuropathy. Ingestion has rarely been reported.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
甲基正丁基酮(MNBK)容易被肺部、胃肠道和皮肤吸收。它不会在呼吸或尿液中大量以未改变的形式排出。从(1-14)C-MNBK派生的放射性物质在人身上缓慢排泄,这表明暴露可能导致长时间的有毒代谢物暴露。
Methyl n-butyl ketone (MNBK) is readily absorbed by lung, gi tract and through skin. It is not eliminated extensively unchanged in breath or urine. Radioactivity derived from (1-14)C-MNBK was excreted slowly by man, suggesting that exposure may lead to prolonged toxic metabolite exposure.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
人类暴露于10或50ppm的甲基正丁基酮(MNBK)7.5小时,或暴露于100ppm的甲基正丁基酮4小时,吸收了吸入蒸汽的75%至92%。两名志愿者通过皮肤接触(1-14)C-MNBK,分别吸收了4.8微克/分钟/平方厘米和8.0微克/分钟/平方厘米。
Humans exposed for 7.5 hr to 10 or 50 ppm or for 4 hr to 100 ppm of methyl n-butyl ketone (MNBK) absorbed between 75 and 92% of inhaled vapor. Two volunteers exposed by skin contact to (1-14)C-MNBK absorbed 4.8 ug/min/sq cm and 8.0 ug/min/sq cm, respectively.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
通过口腔灌胃给大鼠施用放射性标记的MnBK表明,剂量的98%被吸收。比格犬吸入50或100 ppm MnBK 6小时后,吸收了65-68%的蒸气。放射性标记的MnBK通过狗皮肤的吸收一开始很慢,但在暴露20分钟后显著增加...研究结论是,在1小时的皮肤暴露期间,大约会吸收的MnBK量是通过吸入25 ppm MnBK蒸气1小时吸收量的两倍。因此,重复的皮肤暴露于MnBK可能会增加其毒性。同时接触甲基乙基甲酮(MEK)可能会增加MnBK的吸收。
Oral gavage administration of radiolabeled MnBK to rats indicated that 98% of the dose was absorbed. Beagle dogs that inhaled 50 or 100 ppm MnBK for 6 hr absorbed 65-68% of the vapor. absorption of radiolabeled MnBK through dog skin was observed to be slow at first but increased dramatically after 20 min of exposure ... It was concluded that approximately twice as much MnBK would be absorbed during a 1-hr dermal exposure as would be absorbed by inhalation of 25 ppm of MnBK vapor for 1 hr. Therefore, repeated dermal exposure to MnBK may contribute to its toxicity. Concomitant exposure to methyl ethyl ketone (MEK) may increase absorption of MnBK
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
职业暴露等级:B
-
职业暴露限值:TWA: 1 ppm (4 mg/m3)
-
TSCA:Yes
-
危险等级:3
-
立即威胁生命和健康浓度:1,600 ppm
-
危险品标志:F,T
-
安全说明:S16,S36/37,S45,S7
-
危险类别码:R67,R48/23,R10,R11,R23/24/25,R62,R39/23/24/25
-
WGK Germany:1
-
海关编码:29141900
-
危险品运输编号:UN 1224 3/PG 3
-
危险类别:3
-
RTECS号:MP1400000
-
包装等级:II
-
储存条件:将密器密封后,储存在密封的主藏器中,并放置在阴凉、干爽的地方。请远离火源和强氧化剂,避免静电产生。
制备方法与用途
化学性质
这是一种无色液体,熔点为-56.9℃,沸点为127℃,相对密度0.8113(20/4℃),闪点为35℃。它能与乙醇、丙酮和乙醚混溶,并且微溶于水。
用途
此物质用作溶剂和有机合成中间体。易燃,可与空气形成爆炸性混合物,可用于从盐酸溶液中提取三氯化铁以及有机合成。
生产方法
通过将正丙基乙酰乙酸乙酯与15%氢氧化钠溶液共热制得2-己酮。具体步骤为:将这两种物质混合后加热回流6小时,冷却后再加食盐饱和,用乙醚提取。之后再使用食盐水洗涤并以无水氯化钙干燥,回收乙醚,剩余物蒸馏收集127-130℃的馏分。
类别
易燃液体
毒性分级
中毒
急性毒性
口服 - 大鼠 LD50: 2590 毫克/公斤;小鼠 LD50: 710 毫克/公斤
刺激数据
皮肤 - 兔子 500 毫克/24 小时 轻度;眼睛 - 兔子 500 毫克/24小时 轻度
爆炸物危险特性
与空气混合可爆
可燃性危险特性
遇明火、高温或氧化剂易燃,燃烧会产生刺激烟雾
储运特性
应存放在通风低温干燥的库房中,并与其他氧化剂分开存放
职业标准
TLV-TWA 5 PPM (20 毫克/立方米);STEL 8 PPM (35 毫克/立方米)
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,5-己二酮 2,5-hexadione 110-13-4 C6H10O2 114.144 3-己酮 n-hexan-3-one 589-38-8 C6H12O 100.161 环己酮 cyclohexanone 108-94-1 C6H10O 98.1448 5-壬酮 5-Nonanone 502-56-7 C9H18O 142.241 正己醛 hexanal 66-25-1 C6H12O 100.161 1-碘-5-己酮 6-iodohexan-2-one 4367-98-0 C6H11IO 226.057 6-氯-2-己酮 6-chloro-2-hexanone 10226-30-9 C6H11ClO 134.606 1,4-环己二酮 1,4-Cyclohexanedione 637-88-7 C6H8O2 112.128 4-氧代壬-1-醛 4-oxononanal 74327-29-0 C9H16O2 156.225 1-碘己烷-2-酮 1-iodo-2-hexanone 78389-72-7 C6H11IO 226.057 5-已烯-2-酮 1-hexen-5-one 109-49-9 C6H10O 98.1448 5-溴-2-戊酮 bromo-5-pentanone-2 3884-71-7 C5H9BrO 165.03 正丁醛 butyraldehyde 123-72-8 C4H8O 72.1069 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,5-己二酮 2,5-hexadione 110-13-4 C6H10O2 114.144 3-己酮 n-hexan-3-one 589-38-8 C6H12O 100.161 3-庚酮 heptan-3-one 106-35-4 C7H14O 114.188 2,6-庚二酮 2,6-heptandione 13505-34-5 C7H12O2 128.171 环己酮 cyclohexanone 108-94-1 C6H10O 98.1448 5-壬酮 5-Nonanone 502-56-7 C9H18O 142.241 2,4-己二酮 hexane-2,4-dione 3002-24-2 C6H10O2 114.144 —— octadecane-5,14-dione 31335-01-0 C18H34O2 282.467 2,3-己二酮 2,3-hexanedione 3848-24-6 C6H10O2 114.144 2,5-庚二酮 heptane-2,5-dione 1703-51-1 C7H12O2 128.171 —— 3-oxo-heptanal 136119-46-5 C7H12O2 128.171 1-溴己烷-2-酮 1-bromo-2-hexanone 26818-07-5 C6H11BrO 179.057 —— 1-hydroxyhexan-2-one 73397-68-9 C6H12O2 116.16 1-碘己烷-2-酮 1-iodo-2-hexanone 78389-72-7 C6H11IO 226.057 3-甲基-2-己酮 3-methyl-2-hexanone 2550-21-2 C7H14O 114.188 1-庚烯-3-酮 1-hepten-3-one 2918-13-0 C7H12O 112.172 1-氯-2-己酮 1-chlorohexan-2-one 20261-68-1 C6H11ClO 134.606 3-甲基-2-己酮 (S)-(+)-3-methylhexan-2-one 79980-77-1 C7H14O 114.188 2-甲基-5-壬酮 2-methyl-5-nonanone 22287-02-1 C10H20O 156.268 壬烷-2,5-二酮 nonane-2,5-dione 25234-82-6 C9H16O2 156.225 —— 3,6-decanedione 96948-54-8 C10H18O2 170.252 —— 4,7-undecadione 67632-30-8 C11H20O2 184.279 2-甲基-3-庚酮 2-methylheptan-3-one 13019-20-0 C8H16O 128.214 - 1
- 2
- 3
反应信息
-
作为反应物:参考文献:名称:Reetz, Manfred T.; Westermann, Juergen; Steinbach, Rainer, Angewandte Chemie, 1980, vol. 92, # 11, p. 931 - 933摘要:DOI:
-
作为产物:参考文献:名称:Bel'skii; Schuikin, Doklady Akademii Nauk SSSR, 1959, vol. 127, p. 91摘要:DOI:
-
作为试剂:参考文献:名称:一种(E)-烯基砜类化合物的制备方法摘要:本发明公开了一种(E)‑烯基砜类化合物的制备方法:以MeCN‑H2O(3:1)为溶剂,在p‑TsOH存在下,I2和30%H2O2将酮或醛(IV)氧化,原位产生偕二过氧醇类化合物(V),式(V)作为过氧化试剂与I2协同催化芳基烯类化合物(II)与磺酰肼类化合物(III)的反应制备(E)‑烯基砜类化合物(I)。本发明的有益之处在于,过氧化试剂偕二过氧醇类化合物(V)原位产生,操作安全;I2反应后生成的碘离子,在p‑TsOH存在下,被H2O2再氧化原位生成I2,循环参加反应。公开号:CN109096158B
文献信息
-
Enantioselective Transfer Hydrogenation of Aliphatic Ketones Catalyzed by Ruthenium Complexes Linked to the Secondary Face of β-Cyclodextrin作者:Alain Schlatter、Wolf-D. WoggonDOI:10.1002/adsc.200700558日期:2008.5.5Ruthenium-η-arene complexes attached to the secondary face of β-cyclodextrin catalyze the enantioselective reduction (ee up to 98%) of aliphatic and aromatic ketones in aqueous medium in the presence of sodium formate (HCOONa).
-
Separate Sets of Mutations Enhance Activity and Substrate Scope of Amine Dehydrogenase作者:Robert D. Franklin、Conner J. Mount、Bettina R. Bommarius、Andreas S. BommariusDOI:10.1002/cctc.201902364日期:2020.5.7average of 2.5‐fold higher activity toward aliphatic ketones and an 8.0 °C increase in melting temperature. L‐AmDH‐TV did not show significant changes in relative activity for different substrates. In contrast, L39A, L39G, A112G, and T133G in varied combinations added to L‐AmDH‐TV changed the shape of the substrate binding pocket. L‐AmDH‐TV was not active on ketones larger than 2‐hexanone. L39A and L39G
-
Chiral Surfactant-Type Catalyst for Asymmetric Reduction of Aliphatic Ketones in Water作者:Jiahong Li、Yuanfu Tang、Qiwei Wang、Xuefeng Li、Linfeng Cun、Xiaomei Zhang、Jin Zhu、Liangchun Li、Jingen DengDOI:10.1021/ja308357y日期:2012.11.14A novel chiral surfactant-type catalyst is developed. Micelles formed in water by association of the catalysts themselves, and this was confirmed by TEM analyses. Asymmetric transfer hydrogenation of aliphatic ketones catalyzed by the chiral metallomicellar catalyst gave good to excellent conversions and remarkable stereoselectivities (up to 95% ee). Synergistic effects between the metal-catalyzed
-
Novel indole derivatives as selective androgen receptor modulators (SARMS)申请人:Lanter C. James公开号:US20050245485A1公开(公告)日:2005-11-03The present invention is directed to novel indole derivatives, pharmaceutical compositions containing them and their use in the treatment of disorders and conditions modulated by the androgen receptor.
-
Rh(i) and Ir(i) catalysed intermolecular hydroamination with substituted hydrazines
表征谱图
-
氢谱1HNMR
-
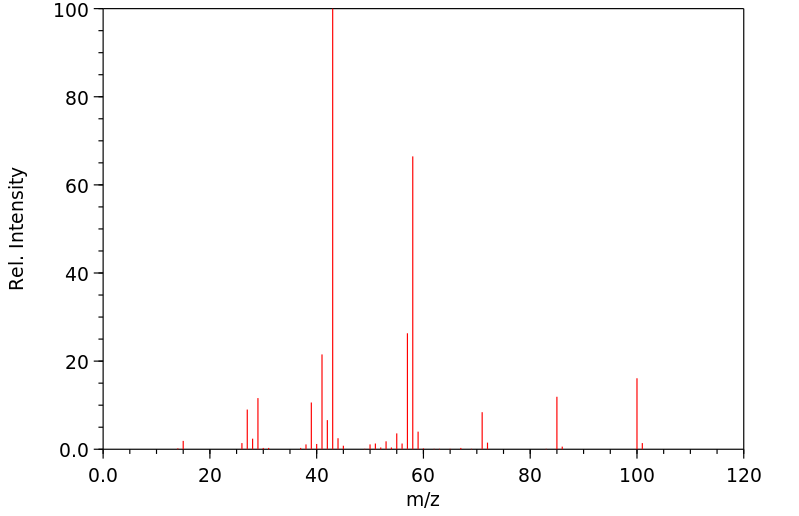
质谱MS
-
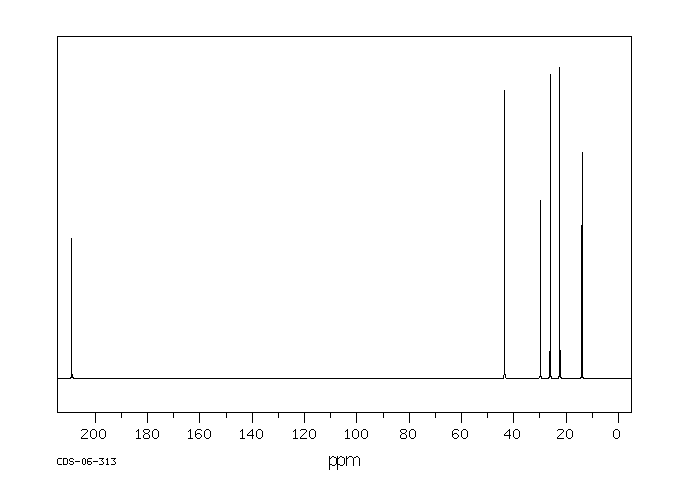
碳谱13CNMR
-
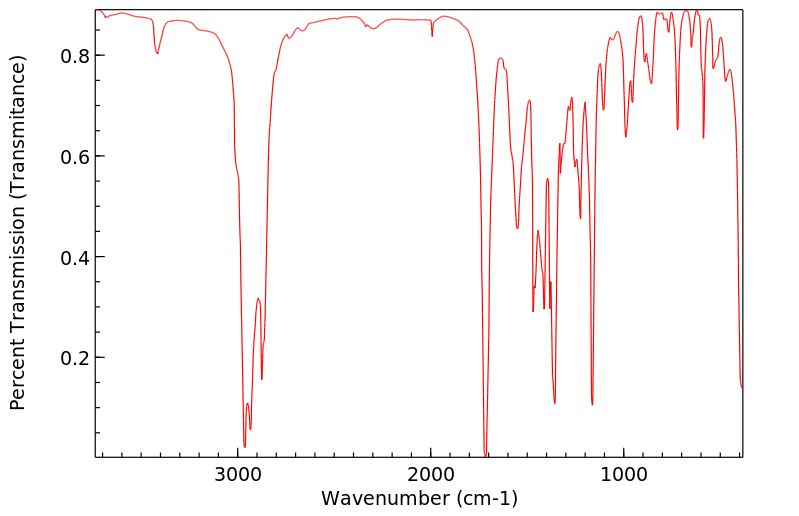
红外IR
-
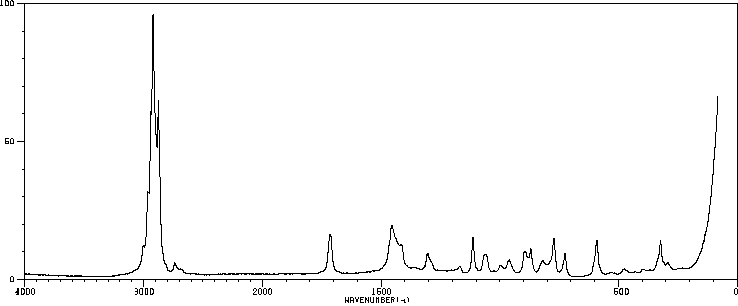
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷