3,4-二甲氧基甲苯 | 494-99-5
中文名称
3,4-二甲氧基甲苯
中文别名
1,2-二甲氧基-4-甲基苯;4-甲基藜芦醚
英文名称
1,2-dimethoxy-4-methylbenzene
英文别名
3,4-dimethoxytoluene;4-methylveratrole;homoveratrole;4-methyl-1,2-dimethoxybenzene
CAS
494-99-5
化学式
C9H12O2
mdl
MFCD00016651
分子量
152.193
InChiKey
GYPMBQZAVBFUIZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:22-23 °C (lit.)
-
沸点:133-135 °C/50 mmHg (lit.)
-
密度:1.051 g/mL at 25 °C (lit.)
-
闪点:185 °F
-
LogP:2.176 (est)
-
保留指数:1171;1195;1204;1198;1199;1201;1204;1174;1207;1207;1208
-
稳定性/保质期:
按规格使用和贮存,不会发生分解,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:18.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S26,S36,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:29093090
-
危险品运输编号:NONH for all modes of transport
-
储存条件:密封保存,并置于通风、干燥的环境中。
SDS
| Name: | 3 4-Dimethoxytoluene 98% Material Safety Data Sheet |
| Synonym: | 4-Methylveratrole |
| CAS: | 494-99-5 |
Synonym:4-Methylveratrole
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 494-99-5 | 3,4-Dimethoxytoluene | 98 | 207-796-0 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Runoff from fire control or dilution water may cause pollution. Combustible liquid and vapor.
Vapors are heavier than air and may travel to a source of ignition and flash back. Vapors can spread along the ground and collect in low or confined areas.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Use a spark-proof tool. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames. Keep away from heat and flame. Avoid breathing vapor or mist.
Storage:
Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 494-99-5: Personal Protective Equipment Eyes: Wear chemical splash goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid or liquid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 133 - 135 deg C @ 50 mmHg
Freezing/Melting Point: 22 - 23 deg C
Autoignition Temperature: Not available.
Flash Point: 85 deg C ( 185.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: Insoluble.
Specific Gravity/Density: 1.0509
Molecular Formula: C9H12O2
Molecular Weight: 152.19
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not currently available.
Conditions to Avoid:
Ignition sources, excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 494-99-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3,4-Dimethoxytoluene - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 494-99-5: No information available.
Canada
CAS# 494-99-5 is listed on Canada's DSL List.
CAS# 494-99-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 494-99-5 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
用途:用作有机合成中间体
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-甲氧基-4-甲基苯酚 2-Methoxy-4-methylphenol 93-51-6 C8H10O2 138.166 3,4-二甲氧基苯甲醛 3,4-dimethoxy-benzaldehyde 120-14-9 C9H10O3 166.177 3,4-二甲氧基苄醇 (3,4-dimethoxyphenyl)methanol 93-03-8 C9H12O3 168.192 3,4-二甲氧基苄基溴 4-bromomethyl-1,2-dimethoxybenzene 21852-32-4 C9H11BrO2 231.089 3,4-二甲氧基苄腈 veratronitrile 2024-83-1 C9H9NO2 163.176 3,4-二甲氧基苄氯 4-chloromethyl-1,2-dimethoxy-benzene 7306-46-9 C9H11ClO2 186.638 3,4-(亚甲二氧基)甲苯 5-methyl-1,3-benzodioxole 7145-99-5 C8H8O2 136.15 香草醛 vanillin 121-33-5 C8H8O3 152.15 3,4-二甲氧基苯乙腈 3,4-dimethoxyphenylacetate 93-17-4 C10H11NO2 177.203 胡椒醇 piperonol 495-76-1 C8H8O3 152.15 —— O-phenyl-3,4-dimethoxybenzyl alcohol 31574-16-0 C15H16O3 244.29 3,4-二羟基甲苯 4-Methylcatechol 452-86-8 C7H8O2 124.139 3,4-二甲氧基苯乙酸 (3,4-Dimethoxyphenyl)acetic acid 93-40-3 C10H12O4 196.203 藜芦基乙酸酯 veratryl acetate 53751-40-9 C11H14O4 210.23 —— 2,4-dimethoxybenzaldehyde semicarbazone 5346-37-2 C10H13N3O3 223.232 1,2-二甲氧基-4-[(2-甲氧基苯氧基)甲基]苯 3,4-dimethoxybenzyl 2-methoxyphenyl ether 10548-82-0 C16H18O4 274.317 邻苯二甲醚 1,2-dimethoxybenzene 91-16-7 C8H10O2 138.166 对甲苯甲醚 4-Methylanisole 104-93-8 C8H10O 122.167 —— 3,4-Dimethoxybenzyl pivalate 157843-80-6 C14H20O4 252.31 胡椒酸 Piperonylic acid 94-53-1 C8H6O4 166.133 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-甲氧基-4-甲基苯酚 2-Methoxy-4-methylphenol 93-51-6 C8H10O2 138.166 2-甲氧基-5-甲基苯酚 5-methyl-2-methoxyphenol 1195-09-1 C8H10O2 138.166 1,2-二甲氧基-4,5-二甲基苯 1,2-dimethoxy-4,5-dimethylbenzene 1128-57-0 C10H14O2 166.22 3,4-二甲氧基苯甲醛 3,4-dimethoxy-benzaldehyde 120-14-9 C9H10O3 166.177 3,4-二甲氧基苄醇 (3,4-dimethoxyphenyl)methanol 93-03-8 C9H12O3 168.192 3,4-二甲氧基苄腈 veratronitrile 2024-83-1 C9H9NO2 163.176 3,4-二甲氧基苄基溴 4-bromomethyl-1,2-dimethoxybenzene 21852-32-4 C9H11BrO2 231.089 —— 1,2-dimethoxy-3,5-dimethylbenzene 30718-65-1 C10H14O2 166.22 2,3-二甲氧基甲苯 1,2-dimethoxy-3-methylbenzene 4463-33-6 C9H12O2 152.193 —— 3,4,4'-Trimethoxy-diphenylmethan 2898-55-7 C16H18O3 258.317 4-[(3,4-二甲氧基苯基)甲基]-1,2-二甲氧基苯 bis(3,4-dimethoxyphenyl)methane 1158-27-6 C17H20O4 288.343 —— 1,2-dimethoxy-3,4-dimethylbenzene 248252-69-9 C10H14O2 166.22 1-乙基-4,5-二甲氧基-2-甲基-苯 3,4-Dimethoxi-6-ethyl-toluol 31465-72-2 C11H16O2 180.247 4,5-二甲氧基-2-甲基苯甲醛 4,5-dimethoxy-2-methylbenzaldehyde 7721-62-2 C10H12O3 180.203 —— α-bromo-4,5-dimethoxy-o-xylene 21852-37-9 C10H13BrO2 245.116 —— 4,5-dimethoxy-2-methylbenzonitrile 58814-69-0 C10H11NO2 177.203 5-羟基-4-甲氧基-2-甲基苯甲醛 2-methyl-4-methoxy-5-hydroxybenzaldehyde 7721-61-1 C9H10O3 166.177 1-(氯甲基)-4,5-二甲氧基-2-甲基苯 1-(chloromethyl)-4,5-dimethoxy-2-methylbenzene 7537-05-5 C10H13ClO2 200.665 4-羟基-5-甲氧基-2-甲基苯甲醛 4-hydroxy-5-methoxy-2-methylbenzaldehyde 42044-81-5 C9H10O3 166.177 3,4-二羟基甲苯 4-Methylcatechol 452-86-8 C7H8O2 124.139 3,4-二甲氧基苯乙酸 (3,4-Dimethoxyphenyl)acetic acid 93-40-3 C10H12O4 196.203 1-溴-4,5-二甲氧基-2-甲基苯 1-bromo-4,5-dimethoxy-2-methylbenzene 52806-46-9 C9H11BrO2 231.089 藜芦酸 Veratric acid 93-07-2 C9H10O4 182.176 —— 5-Mercapto-4-methyl-veratrol 78523-20-3 C9H12O2S 184.259 1-甲基-4,5-二甲氧基-2-氯苯 6-chloro-3,4-dimethoxytoluene 33963-29-0 C9H11ClO2 186.638 (3,4-二甲氧基苯基)乙酰氯 3,4-dimethoxyphenylacetyl chloride 10313-60-7 C10H11ClO3 214.649 1-碘-4,5-二甲氧基-2-甲基苯 1-iodo-4,5-dimethoxy-2-methylbenzene 116131-35-2 C9H11IO2 278.09 藜芦基乙酸酯 veratryl acetate 53751-40-9 C11H14O4 210.23 —— 4,5-dimethoxy-2-methylbenzyl cyanide 7537-09-9 C11H13NO2 191.23 3,4-二甲氧基苯甲酰胺 veratramide 1521-41-1 C9H11NO3 181.191 —— 2-Methyl-4.5-dimethoxyphenethylamin 94823-73-1 C11H17NO2 195.261 —— 1,3-bis(3,4-dimethoxyphenyl)-propan-2-one 6704-25-2 C19H22O5 330.381 1-溴-2,3-二甲氧基-5-甲基苯 3-bromo-4,5-dimethoxy-toluene 68278-85-3 C9H11BrO2 231.089 —— (p-anisyl)(4,5-dimethoxy-2-methylphenyl)methane —— C17H20O3 272.344 —— (4,5-dimethoxy-2-methylphenyl)-(3,4-dimethoxyphenyl)-methane 102415-87-2 C18H22O4 302.37 —— 4-((E)-4,8-Dimethyl-nona-3,7-dienyl)-1,2-dimethoxy-benzene 106625-40-5 C19H28O2 288.43 —— bis-(4,5-dimethoxy-2-methyl-phenyl)-methane 1164-05-2 C19H24O4 316.397 —— 2-chloro-3,4-dimethoxytoluene —— C9H11ClO2 186.638 3,4-二甲氧基苯甲酸甲酯 methyl 3,4-dimethoxybenzoate 2150-38-1 C10H12O4 196.203 —— N-(3,4-dimethoxybenzylidene)phenylhydrazine 55754-32-0 C15H16N2O2 256.304 —— 4-(3,4-dimethoxybenzyl)morpholine 22792-30-9 C13H19NO3 237.299 —— (S)-tert-butyl (3,4-dimethoxyphenyl)methyl sulfoxide 1361250-90-9 C13H20O3S 256.366 2-溴-4,5-二甲氧基溴苄 1-bromo-2-(bromomethyl)-4,5-dimethoxybenzene 53207-00-4 C9H10Br2O2 309.985 4-(4,5-二甲氧基-2-甲基-苯基)-丁酸 4-<2-Methyl-4,5-dimethoxy-phenyl>-buttersaeure 6575-52-6 C13H18O4 238.284 —— 1-(4,5-Dimethoxy-2-methyl-phenyl)-propan-2-one 7652-98-4 C12H16O3 208.257 —— (4,5-Dimethoxy-2-methyl-phenyl)-propynoic acid 131035-53-5 C12H12O4 220.225 —— (E)-3-(4,5-dimethoxy-2-methyl-phenyl)prop-2-enoic acid 105253-94-9 C12H14O4 222.241 —— 4,5-Dimethoxy-2-methyl-β-nitro-styrol 64372-66-3 C11H13NO4 223.229 —— 4,5-dimethoxy-2-methylbenzoic acid 131035-49-9 C10H12O4 196.203 —— 4,5-dimethoxy-2-methyl-benzaldehyde-semicarbazone 62036-33-3 C11H15N3O3 237.258 - 1
- 2
- 3
- 4
- 5
反应信息
-
作为反应物:描述:3,4-二甲氧基甲苯 在 lithium hydroxide 、 N-溴代丁二酰亚胺(NBS) 、 lithium diisopropyl amide 作用下, 以 四氢呋喃 、 甲醇 、 氯仿 、 N,N-二甲基甲酰胺 为溶剂, 反应 29.0h, 生成 (S)-(-)-3-[2-(2-hydroxyethyl)-4,5-dimethoxyphenyl]-6,7-dimethoxy-3,4-dihydro-2H-isoquinolin-1-one参考文献:名称:使用对映体纯的亚磺胺类药物合成小ber碱生物碱(S)-(-)-木吡啶碱的不对称合成。摘要:描述了(S)-(-)-草氨吡啶(1)的简明对映选择性合成,该方法涉及在对映纯的亚氨嘧啶(+)-14中添加邻甲苯二异氰酸酯16的侧锂基化衍生物。用DIBAL-H处理所得的氰基亚磺酰胺加合物(-)-17b,可在一个罐中完成五次操作,并以良好的收率得到环状亚胺(+)-18。还原和环化得到(S)-(-)-1。或者,17b,c的碱性水解得到异喹诺酮21,其被环化并还原成(S)-(-)-1。DOI:10.1021/jo010988v
-
作为产物:描述:罂粟碱 生成 3,4-二甲氧基甲苯参考文献:名称:Goldschmiedt, Monatshefte fur Chemie, 1883, vol. 4, p. 705摘要:DOI:
-
作为试剂:描述:benzyl 2-pyrimidine sulfide 在 (18)F-KF-Kryptofix 2.2.2 complex 、 3,4-二甲氧基甲苯 作用下, 以 四氢呋喃 、 二氯甲烷 为溶剂, 反应 0.33h, 生成 氟甲苯参考文献:名称:[EN] COMPOUNDS AND KITS FOR PREPARING IMAGING AGENTS AND METHODS OF IMAGING
[FR] COMPOSES ET KITS DE PREPARATION D'AGENTS D'IMAGERIE ET PROCEDES D'IMAGERIE摘要:将包括与一个区域选择性离去基团结合的靶向基团的化合物用于制备成像剂是有用的。这些成像剂可以通过固体支持物来分离出来,基于分子的化学属性(例如净电荷或极性)或物理属性之间的差异,以此与离去基团衍生的副产物进行区分。制备成像剂的方法包括以下步骤:提供一个包含通过链连接基团与支持物相结合的靶向基团的化合物,该链连接基团包含一个用于区域选择性取代可检测物种的位点;将该化合物与含有可检测物种的溶液接触;并回收成像剂。还有一种用于制备成像剂的工具包,其中包括一个第一容器(5),其中含有一个含有可检测物种的溶液(31),以及一个第二容器(2),其中含有一个通过含有一个用于区域选择性取代可检测物种的离去基团的位点而与支持物(30)相结合的靶向基团的化合物。公开号:WO2005023317A1
文献信息
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
[EN] SUBSTITUTED QUINAZOLINES AS FUNGICIDES<br/>[FR] QUINAZOLINES SUBSTITUÉES, UTILISÉES EN TANT QUE FONGICIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2010136475A1公开(公告)日:2010-12-02The present invention relates to a compound of formula (I) wherein wherein the substituents have the definitions as defined in claim 1or a salt or a N-oxide thereof, their use and methods for the control and/or prevention of microbial infection, particularly fungal infection, in plants and to processes for the preparation of these compounds.本发明涉及一种具有如下式(I)的化合物,其中取代基具有权利要求1中定义的定义,或其盐或N-氧化物,它们的用途以及用于控制和/或预防植物中微生物感染,特别是真菌感染的方法,以及制备这些化合物的方法。
-
Active Molybdenum-Based Anode for Dehydrogenative Coupling Reactions作者:Sebastian B. Beil、Timo Müller、Sydney B. Sillart、Peter Franzmann、Alexander Bomm、Michael Holtkamp、Uwe Karst、Wolfgang Schade、Siegfried R. WaldvogelDOI:10.1002/anie.201712718日期:2018.2.23and powerful active anode system that can be operated in 1,1,1,3,3,3‐hexafluoro‐2‐propanol (HFIP) has been discovered. In HFIP the molybdenum anode forms a compact, conductive, and electroactive layer of higher‐valent molybdenum species. This system can replace powerful but stoichiometrically required MoV reagents for the dehydrogenative coupling of aryls. This electrolytic reaction is more sustainable发现了一种可以在1,1,1,3,3,3-六氟-2-丙醇(HFIP)中运行的功能强大的新型阳极活性系统。在HFIP中,钼阳极形成一个高价钼物种的致密,导电和电活性层。该系统可替代功能强大但化学计量上要求的Mo V试剂,用于芳基的脱氢偶联。这种电解反应是更可持续的,并允许转化广泛范围的活化芳烃。
-
Triazolone derivatives申请人:Clark Richard公开号:US20080015199A1公开(公告)日:2008-01-17A Compound represented by the following general formula (1), salts thereof or hydrates of the foregoing is a novel compound useful for treatment and/or prevention of diseases associated with thrombus formation, and which is safer with suitable physicochemical stability. [wherein R 1a , R 1b , R 1c and R 1d each independently represent hydrogen, etc.; R 2 represents optionally substituted phenyl, etc.; R 3 represents optionally substituted C6-10 aryl, etc.; and Z 1 and Z 2 each independently represent hydrogen]
-
Can Heteroarenes/Arenes Be Hydrogenated Over Catalytic Pd/C Under Ambient Conditions?作者:Nao Tanaka、Toyonobu UsukiDOI:10.1002/ejoc.202000695日期:2020.9.14Pd/C‐mediated dearomatic hydrogenation under ambient conditions such as balloon pressure and room temperature can be a powerful tool for constructing alicyclic skeletons. Density functional theory calculations have been performed to confirm the mechanistic aspects and the utility of the established methodology has been demonstrated by donepezil synthesis.在诸如气球压力和室温等环境条件下,Pd / C介导的脱芳香族氢化反应可能是构建脂环族骨架的强大工具。已经进行了密度泛函理论计算以确认机理方面,并且通过多奈哌齐合成证明了所建立方法的实用性。
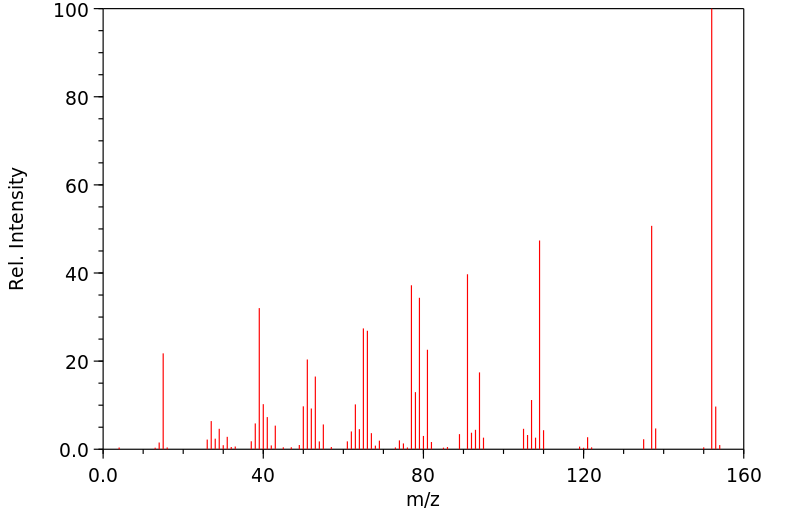
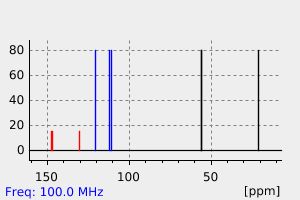
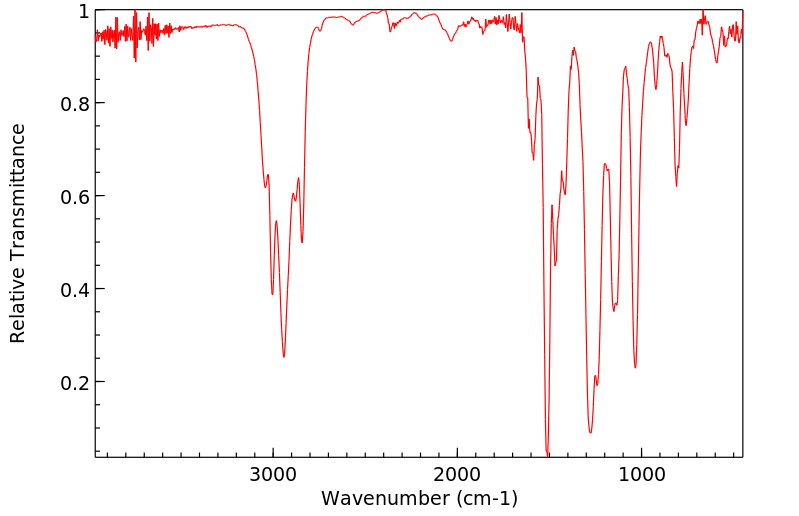
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫