4-(9H-咔唑-9-基)苯硼酸 | 419536-33-7
中文名称
4-(9H-咔唑-9-基)苯硼酸
中文别名
4-(9-咔唑基)苯硼酸;B-[4-(9H-咔唑-9-基)苯基]硼酸;4-(9H-咔唑)苯硼酸;4-(9-咔唑基)苯基硼酸
英文名称
4-(carbazol-9-yl)phenylboronic acid
英文别名
4-(9H-carbazol-9-yl)phenylboronic acid;4-(9-carbazolyl)phenylboronic acid;4-(9H-carbazol-9-yl)benzeneboronic acid;4-(N-carbazolyl)phenylboronic acid;4-(9H-carbazole-9-yl)phenylboronic acid;4-(9H-carbazolyl)phenyl-boronic acid;4-(9-carbazolyl)benzeneboronic acid;4-(N-carbazolyl)benzeneboronic acid;4-(9-carbazole)phenylboronic acid;4-carbazolyl-1-phenylboronic acid;4-(9H-carbozol-9-yl)phenylboronic acid;9H-carbazole-9-(4-phenyl)boronic acid;4-(carbazol-9-yl)benzeneboronic acid;4-(carbazole-9-yl)phenylboronic acid;(4-(9H-Carbazol-9-yl)phenyl)boronic acid;(4-carbazol-9-ylphenyl)boronic acid
CAS
419536-33-7
化学式
C18H14BNO2
mdl
——
分子量
287.126
InChiKey
JGAVTCVHDMOQTJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:264°C(lit.)
-
沸点:452.7±51.0 °C(Predicted)
-
密度:1.20±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.46
-
重原子数:22
-
可旋转键数:2
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:45.4
-
氢给体数:2
-
氢受体数:2
安全信息
-
海关编码:2933990090
-
危险性防范说明:P261,P280,P305+P351+P338,P304+P340,P405,P501
-
危险性描述:H315,H319,H335
-
储存条件:室温且干燥
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 4-(9H-Carbozol-9-yl)phenylboronic acid
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-(9H-Carbozol-9-yl)phenylboronic acid
CAS number: 419536-33-7
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C18H14BNO2
Molecular weight: 287.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 4-(9H-Carbozol-9-yl)phenylboronic acid
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-(9H-Carbozol-9-yl)phenylboronic acid
CAS number: 419536-33-7
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C18H14BNO2
Molecular weight: 287.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 9-(4-溴苯基)咔唑 N-(4-bromophenyl)carbazole 57102-42-8 C18H12BrN 322.204 9-(4-氯苯基)咔唑 9-(4-chlorophenyl)-9H-carbazole 19264-71-2 C18H12ClN 277.753 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-苯基咔唑 N-phenylcarbazole 1150-62-5 C18H13N 243.308 9-(4-碘苯基)咔唑 9-(4-iodophenyl)carbazole 57103-15-8 C18H12IN 369.204 —— 4-(9H-carbazol-9-yl)phenol 222620-05-5 C18H13NO 259.307 4-(9-咔唑)基-4苯胺 4-(9H-carbazol-9-yl)aniline 52708-37-9 C18H14N2 258.323 4,4'-二(9-咔唑)联苯 4,4'-bis(9H-carbazol-9-yl)biphenyl 58328-31-7 C36H24N2 484.6 9-[4-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)苯基]-9H-咔唑 9-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-9H-carbazole 785051-54-9 C24H24BNO2 369.271 —— 4'-(9H-carbazol-9-yl)biphenyl-2-boronic acid 1215228-59-3 C24H18BNO2 363.223 9-(4'-溴联苯-4-基)-9H-咔唑 9-(4'-bromo-[1,1'-biphenyl]-4-yl)-9H-carbazole 212385-73-4 C24H16BrN 398.302 —— 9,9'-(1,1':3',1''-terphenyl-4,4''-diyl)dicarbazole 910647-25-5 C42H28N2 560.698 1,3,5-三[4-(9-咔唑基)苯基]苯 1,3,5-tris[4-(N-carbazolyl)phenyl]benzene 160780-82-5 C60H39N3 801.99 —— 9-(3'-bromo-[1,1'-biphenyl]-4-yl)-9H-carbazole 854952-47-9 C24H16BrN 398.302 —— 9-(4-(pyrimidin-5-yl)phenyl)-9H-carbazole 1380225-74-0 C22H15N3 321.381 —— 9-(4-(methylsulfonyl)phenyl)-9H-carbazole —— C19H15NO2S 321.4 9-(2'-溴-4-联苯基)咔唑 9-(2'-bromobiphenyl-4-yl)-9H-carbazole 1215228-57-1 C24H16BrN 398.302 —— 3-(4-(9H-carbazol-9-yl)phenyl)-9H-carbazole 1060735-25-2 C30H20N2 408.502 —— 9,9'-(1,1':2',1''-terphenyl-4,4''-diyl)dicarbazole 884302-97-0 C42H28N2 560.698 —— 3,6-bis(4-carbazol-9-yl-phenyl)-9H-carbazole 1060735-27-4 C48H31N3 649.794 —— 4'-(carbazol-9-yl)-N-phenyl-[1,1'-biphenyl]-4-amine 331980-55-3 C30H22N2 410.518 —— 9-[4-(6-methoxynaphthalen-2-yl)-phenyl]-9H-carbazole 1229902-32-2 C29H21NO 399.492 —— 4,4'-bis[4-(9H-carbazol-9-yl)phenyl]-1,1'-binaphthyl 1246261-64-2 C56H36N2 736.915 —— (E)-4,4'-bis[4-(carbazol-9-yl)phenyl]stilbene 1020847-64-6 C50H34N2 662.833 —— CzPS 1020847-67-9 C32H23N 421.541 —— 9-(4-(10-phenylanthracen-9-yl)phenyl)-9H-carbazole 910647-23-3 C38H25N 495.623 —— 9-(4-(9,10-diphenylanthracen-7-yl)phenyl)-9H-carbazole 1202059-35-5 C44H29N 571.721 —— 9-(4-(pyridin-2-yl)phenyl)-9H-carbazole 947192-78-1 C23H16N2 320.393 —— 9-(4-(quinolin-3-yl)phenyl)-9H-carbazole 1380225-77-3 C27H18N2 370.453 —— 1,3,6,8-tetrakis(4-(9H-carbazol-9-yl)phenyl)pyrene 1337909-81-5 C88H54N4 1167.42 —— 9-[4-(5-Bromopyridin-3-yl)phenyl]carbazole 1082549-95-8 C23H15BrN2 399.289 —— 2,4,6-tris(4-(9H-carbazol-9-yl)phenyl)-1,3,5-triazine 1393350-75-8 C57H36N6 804.953 9-(4-(4,6-二苯基-1,3,5-三嗪-2-基)苯基)-9H-咔唑 9-(4-(4,6-diphenyl-1,3,5-triazin-2-yl)phenyl)-9H-carbazole 440354-93-8 C33H22N4 474.564 —— 9-(4-(pyrazin-2-yl)phenyl)-9H-carbazole 1380225-75-1 C22H15N3 321.381 —— 9,10-di(4-carbazol-9-ylphenyl)-phenanthrene 1010812-14-2 C50H32N2 660.817 9-(4-喹啉-2-基苯基)咔唑 9-(4-(quinolin-2-yl)phenyl)-9H-carbazole 901117-84-8 C27H18N2 370.453 —— 4-[3,5-bis(4-carbazol-9-ylphenyl)phenyl]-N,N-diphenylaniline 1097915-67-7 C60H41N3 804.006 —— 2,2'-bis[4-(9H-carbazol-9-yl)phenyl]-4,4'-biphenyl diamine 1236224-35-3 C48H34N4 666.825 —— 9-(4-(6-fluoropyridin-2-yl)phenyl)-9H-carbazole 1380225-69-3 C23H15FN2 338.384 —— di(4-(4-(carbazol-9-yl)phenyl)phenyl)sulfone 1443674-65-4 C48H32N2O2S 700.86 —— 9-(4-(6-methoxypyridin-3-yl)phenyl)-9H-carbazole 1380225-76-2 C24H18N2O 350.42 —— 9-(4-(5-fluoropyridin-2-yl)phenyl)-9H-carbazole 1380225-66-0 C23H15FN2 338.384 —— 9-[4-(4-Methylpyridin-2-yl)phenyl]carbazole 1617519-02-4 C24H18N2 334.42 —— 9-(4-(5-methylpyridin-2-yl)phenyl)-9H-carbazole 1380225-65-9 C24H18N2 334.42 —— 1,4-bis-[4-(9-carbazolyl)phenyl]durene 1399362-58-3 C46H36N2 616.805 —— 9-[4-(isoquinolin-1-yl)phenyl]-9H-carbazole 1395928-53-6 C27H18N2 370.453 —— 2,5-bis(4-(9H-carbazol-9-yl)phenyl)pyridine 1029944-02-2 C41H27N3 561.685 —— 9-[4-[4-[1-[4-(4-Carbazol-9-ylphenyl)phenyl]-2,2-diphenylethenyl]phenyl]phenyl]carbazole 1364425-42-2 C62H42N2 815.029 —— 2-[4-(4-(9H-carbazol-9-yl)-phenyl)-phenyl]-5-[4-(9H-carbazol-9-yl)-phenyl]-pyridine 1309356-43-1 C47H31N3 637.783 —— 9-(4-(6-methylpyridin-2-yl)phenyl)-9H-carbazole 1380225-68-2 C24H18N2 334.42 —— 3,8-bis[4-(9H-carbazol-9-yl)phenyl]-1,10-phenanthroline 888026-72-0 C48H30N4 662.793 —— 9-[4-(2-Chloropyrimidin-4-yl)phenyl]carbazole 1419577-24-4 C22H14ClN3 355.826 —— 7-(4-Carbazol-9-ylphenyl)benzo[f][4,7]phenanthroline —— C34H21N3 471.561 —— 4-[4-(9H-carbazol-9-yl)phenyl]-4'-(9,10-diphenyl-2-anthryl)triphenylamine 1404222-74-7 C62H42N2 815.029 —— 9-(3'-(4,6-diphenyl-1,3,5-triazin-2-yl)-[1,1'-biphenyl]-4-yl)-9H-carbazole 864377-32-2 C39H26N4 550.662 —— 9-(4-(6-methoxypyridin-2-yl)phenyl)-9H-carbazole 1380225-72-8 C24H18N2O 350.42 —— 9-[4-[3,5-Di(dibenzofuran-2-yl)phenyl]phenyl]carbazole 1098257-66-9 C48H29NO2 651.764 —— 9-[4-[2,6-Bis(4-carbazol-9-ylphenyl)pyridin-4-yl]phenyl]carbazole 1416368-47-2 C59H38N4 802.978 —— 6-(4-(9H-carbazol-9-yl)phenyl)picolinonitrile 1380225-73-9 C24H15N3 345.403 —— 7-(4-(9H-carbazol-9-yl)phenyl)-3-phenyl-3,10-dihydropyrrolo[3,2-a]carbazole —— C38H25N3 523.637 —— 6-(4-(9H-carbazol-9-yl)phenyl)picolinaldehyde 1380225-70-6 C24H16N2O 348.404 —— 9-(4-(5-(trifluoromethyl)pyridin-2-yl)-phenyl)-9H-carbazole 1617519-03-5 C24H15F3N2 388.392 —— 7-(4-Carbazol-9-ylphenyl)-3,10-diphenylpyrrolo[3,2-a]carbazole —— C44H29N3 599.734 —— 9-(4-(5-nitropyridin-2-yl)phenyl)-9H-carbazole 1380225-67-1 C23H15N3O2 365.391 —— 2,7-bis[4-(9-carbazolyl)phenyl]-[1]benzothieno[3,2-b][1]benzothiophene 1162669-61-5 C50H30N2S2 722.934 - 1
- 2
- 3
- 4
- 5
- 6
- 7
反应信息
-
作为反应物:描述:4-(9H-咔唑-9-基)苯硼酸 在 aluminum (III) chloride 、 四(三苯基膦)钯 、 potassium fluoride dihydrate 作用下, 以 四氢呋喃 、 二氯甲烷 、 水 、 二甲基亚砜 为溶剂, 反应 12.5h, 生成 N-(7-溴-9,9-二甲基芴-2-基)咔唑参考文献:名称:光电材料中间体N-(7-溴-9,9-二甲基芴-2-基)咔唑的合成方法摘要:本发明属于有机合成领域,涉及光电材料中间体N‑(7‑溴‑9,9‑二甲基芴‑2‑基)咔唑的合成。该方法将4‑咔唑基苯硼酸和5‑溴‑2‑碘苯甲酸甲酯偶联,再用格氏试剂生成甲基化产物,最后用三氯化铝傅克反应关环,得到目标产物。该方法通过降低反应温度,提高溴碘的选择性,提高了纯度和收率,收率达87%以上。降低了成本,适合工业化化生产。公开号:CN112194612A
-
作为产物:参考文献:名称:Novel multifunctional organic semiconductor materials based on 4,8-substituted 1,5-naphthyridine: synthesis, single crystal structures, opto-electrical properties and quantum chemistry calculation摘要:一系列4,8取代的1,5-萘啶(1a–1h)已成功合成,通过4,8-二溴-1,5-萘啶(4)与相应的硼酸(2a–2h)在催化性醋酸钯的存在下进行铃木交叉偶联反应,收率为41.4%–75.8%,并得到良好表征。它们在热稳定性方面表现优异,具有高相变温度(高于186°C)。化合物1b、1e和1f分别在单斜晶系中结晶,空间群为P21/c、P21/c和P21/n。所有化合物表现出最低能量吸收带(λmaxAbs: 294–320 nm),揭示了较低的光学带隙(2.77–3.79 eV)。这些材料在二氯甲烷稀溶液中发出蓝色荧光,λmaxEm范围为434–521 nm,在固态中为400–501 nm。4,8取代的1,5-萘啶1a–1h的估计电子亲合能(EA)为2.38–2.72 eV,适合用作电子传输材料,而离子化势(IP)为4.85–5.04 eV,促进了优良的孔注入/孔传输材料特性。使用DFT B3LYP/6-31G*进行的量子化学计算显示,最低未占分子轨道(LUMO)几乎相同,范围为−2.39至−2.19 eV,最高占分子轨道(HOMO)范围为−5.33至−6.84 eV。这些结果表明,具有简单结构的4,8取代1,5-萘啶1a–1h可能是开发高效OLED的有希望的蓝色发射(或蓝绿色发射)材料、电子传输材料以及孔注入/孔传输材料。DOI:10.1039/c2ob25926e
-
作为试剂:描述:9-(4-溴苯基)咔唑 、 n-butyllithium hexane 、 硼酸三甲酯 、 盐酸 在 氮 、 乙酸乙酯 、 Brine 、 magnesium sulfate 、 氯仿 、 正己烷 、 white powder 、 4-(9H-咔唑-9-基)苯硼酸 作用下, 以 四氢呋喃 为溶剂, 反应 28.0h, 以was obtained in yield 58%的产率得到4-(9H-咔唑-9-基)苯硼酸参考文献:名称:Anthracene Derivatives and Light-Emitting Devices Using the Anthracene Derivatives摘要:本发明提供了新型蒽衍生物。特别地,本发明提供具有高发光效率的发光元件和具有长寿命的发光元件。此外,本发明通过使用这些发光元件,提供了具有长寿命的发光器件和电子器件。提供了由通式(1)表示的蒽衍生物。此外,由于通式(1)表示的蒽衍生物具有高发光效率,因此使用该蒽衍生物的发光元件也可以具有高发光效率。通过使用通式(1)表示的蒽衍生物,可以提供具有长寿命的发光元件。公开号:US20090004506A1
文献信息
-
NiH‐Catalyzed Migratory Defluorinative Olefin Cross‐Coupling: Trifluoromethyl‐Substituted Alkenes as Acceptor Olefins to Form <i>gem</i> ‐Difluoroalkenes作者:Fenglin Chen、Xianfeng Xu、Yuli He、Genping Huang、Shaolin ZhuDOI:10.1002/anie.201915840日期:2020.3.23a NiH-catalyzed migratory defluorinative coupling between two electronically differentiated olefins. A broad range of unactivated donor olefins can be joined directly to acceptor olefins containing an electron-deficient trifluoromethyl substituent in both intra- and intermolecular fashion to form gem-difluoroalkenes. This migratory coupling shows both site- and chemoselectivity under mild conditions
-
화합물, 이를 포함하는 유기 광전자 소자 및 표시장치申请人:CHEIL INDUSTRIES INC. 제일모직주식회사(119980034532) Corp. No ▼ 170111-0000076BRN ▼504-81-00025公开号:KR20150130221A公开(公告)日:2015-11-23하기 화학식 1로 표시되는 화합물, 이를 포함하는 유기 광전자 소자 및 상기 유기 광전자 소자를 포함하는 표시장치에 관한 것이다. [화학식 1] 상기 화학식 1에서, L 내지 L, R 내지 R의 정의는 명세서에 정의된 바와 같다.
-
Palladium-catalyzed Suzuki–Miyaura reaction of fluorinated vinyl chloride: a new approach for synthesis α and α,β-trifluoromethylstyrenes作者:Yang Li、Bo Zhao、Kun Dai、Dong-Huai Tu、Bo Wang、Yao-Yu Wang、Zhao-Tie Liu、Zhong-Wen Liu、Jian LuDOI:10.1016/j.tet.2016.07.085日期:2016.9A mild and efficient palladium-catalyzed cross-coupling between fluorinated vinyl chloride and arylboronic acids is described. The use of ligand B successfully overcomes the strong electronic withdrawing of trifluoromethylated substrates and allows the efficient synthesis of a wide range of α and α,β-trifluoromethyl containing olefins. By using this method, the key intermediate for synthesis of Efavirenz
-
Oxidative Heck Reaction of Fluorinated Olefins with Arylboronic Acids by Palladium Catalysis作者:Yang Li、Dong-Huai Tu、Yu-Jie Gu、Bo Wang、Yao-Yu Wang、Zhao-Tie Liu、Zhong-Wen Liu、Jian LuDOI:10.1002/ejoc.201500597日期:2015.7The palladium-catalyzed oxidative Heck reaction of 2,3,3,3-tetrafluoroprop-1-ene with various arylboronic acids was explored for the first time. This method provides a direct route to access (Z)-β-fluoro-β-(trifluoromethyl)styrenes with high stereoselectivity.
-
Promoting charge separation in donor–acceptor conjugated microporous polymers <i>via</i> cyanation for the photocatalytic reductive dehalogenation of chlorides作者:Weijie Zhang、Jiyong Deng、Zhengjun Fang、Donghui Lan、Yunfeng Liao、Xiang Zhou、Qingquan LiuDOI:10.1039/d1cy01386f日期:——Conjugated microporous polymers (CMPs) have emerged as promising heterogeneous photocatalysts for organic transformations owing to their structural designability and functional versatility. However, limited by the insufficient separation of the photo-generated excitons, their photocatalytic efficiency falls far short of expectations. Herein, we demonstrate a cyanation strategy to promote charge carrier共轭微孔聚合物(CMPs)由于其结构可设计性和功能多功能性,已成为有前途的有机转化多相光催化剂。然而,受光生激子分离不充分的限制,其光催化效率远低于预期。在此,我们展示了一种氰化策略,通过选择性地将咔唑和氰基分别作为供电子和吸电子单元来促进 CMP 中的电荷载流子分离。由此产生的 CMP 具有 π 扩展的供体 (D)-受体 (A) 共轭结构,赋予它们独特的半导体特性,其中促进了有效的电荷分离和转移以及广泛的可见光吸收。与无氰对应物相比,氰基官能化的 CMP 显示出优异的光催化效率,例如氯化物的光催化还原脱卤。更突出的是,所设计的 CMP 的完全可回收性以及至少 10 次运行的催化活性而不会损失在氯化物的光催化还原脱卤中的催化性能,这证明了它们的稳健性和可持续性。
表征谱图
-
氢谱1HNMR
-
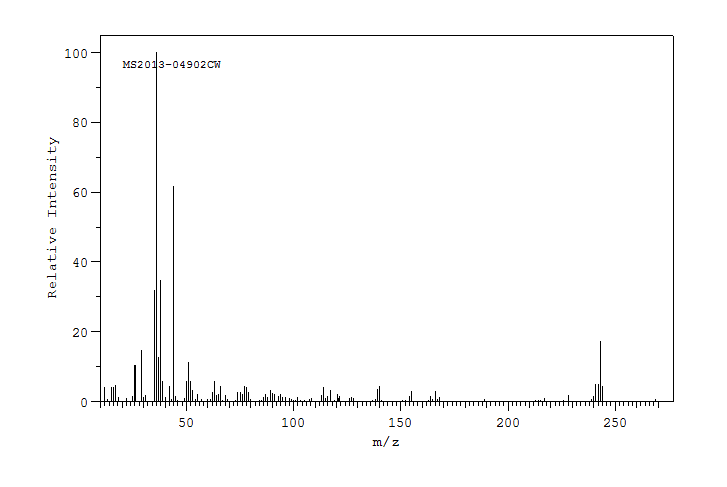
质谱MS
-
碳谱13CNMR
-
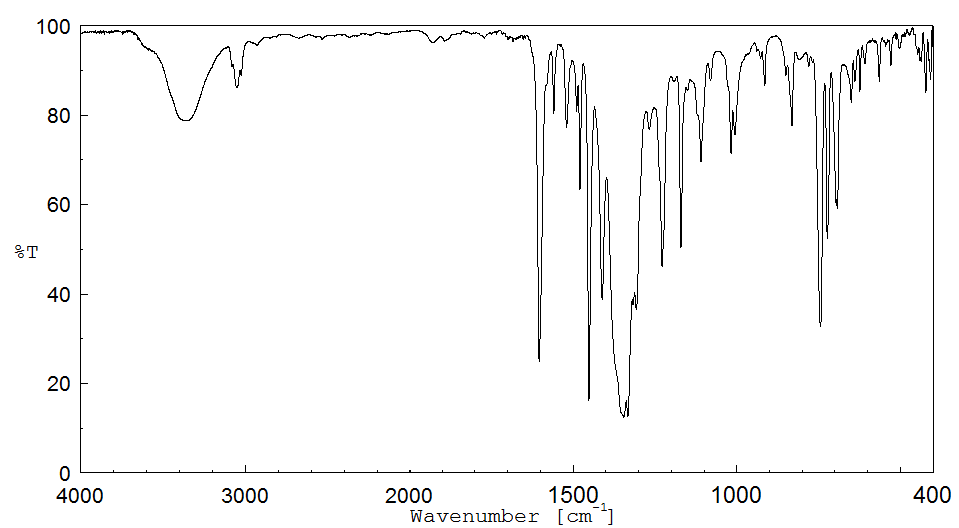
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Z)-3-[[[2,4-二甲基-3-(乙氧羰基)吡咯-5-基]亚甲基]吲哚-2--2-
(S)-(-)-5'-苄氧基苯基卡维地洛
(R)-(+)-5'-苄氧基卡维地洛
(R)-卡洛芬
(N-(Boc)-2-吲哚基)二甲基硅烷醇钠
(E)-2-氰基-3-(5-(2-辛基-7-(4-(对甲苯基)-1,2,3,3a,4,8b-六氢环戊[b]吲哚-7-基)-2H-苯并[d][1,2,3]三唑-4-基)噻吩-2-基)丙烯酸
(4aS,9bR)-6-溴-2,3,4,4a,5,9b-六氢-1H-吡啶并[4,3-B]吲哚
(3Z)-3-(1H-咪唑-5-基亚甲基)-5-甲氧基-1H-吲哚-2-酮
(3Z)-3-[[[4-(二甲基氨基)苯基]亚甲基]-1H-吲哚-2-酮
(3R)-(-)-3-(1-甲基吲哚-3-基)丁酸甲酯
(3-氯-4,5-二氢-1,2-恶唑-5-基)(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙酸
齐多美辛
鸭脚树叶碱
鸭脚木碱,鸡骨常山碱
鲜麦得新糖
高氯酸1,1’-二(十六烷基)-3,3,3’,3’-四甲基吲哚碳菁
马鲁司特
马鞭草(VERBENAOFFICINALIS)提取物
马来酸阿洛司琼
马来酸替加色罗
顺式-ent-他达拉非
顺式-1,3,4,4a,5,9b-六氢-2H-吡啶并[4,3-b]吲哚-2-甲酸乙酯
顺式-(+-)-3,4-二氢-8-氯-4'-甲基-4-(甲基氨基)-螺(苯并(cd)吲哚-5(1H),2'(5'H)-呋喃)-5'-酮
靛青二磺酸二钾盐
靛藍四磺酸
靛红联二甲酚
靛红磺酸钠
靛红磺酸
靛红乙烯硫代缩酮
靛红-7-甲酸甲酯
靛红-5-磺酸钠
靛红-5-磺酸
靛红-5-硫酸钠盐二水
靛红-5-甲酸甲酯
靛红
靛玉红衍生物E804
靛玉红3'-单肟5-磺酸
靛玉红-3'-单肟
靛玉红
靛噻
青色素3联己酸染料,钾盐
雷马曲班
雷莫司琼杂质13
雷莫司琼杂质12
雷莫司琼杂质
雷替尼卜定
雄甾-1,4-二烯-3,17-二酮
阿霉素的代谢产物盐酸盐
阿贝卡尔
阿西美辛杂质3