4-溴苯甲酰苯 | 90-90-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:79-84 °C(lit.)
-
沸点:350 °C(lit.)
-
密度:1.4245 (rough estimate)
-
闪点:350°C
-
保留指数:1841
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:15
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2914700090
-
危险品运输编号:NONH for all modes of transport
-
RTECS号:DJ0350000
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:室温、干燥且密封保存。
SDS
模块 1. 化学品
产品名称: 4-Bromobenzophenone
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 4-溴二苯甲酮
百分比: >98.0%(GC)
CAS编码: 90-90-4
分子式: C13H9BrO
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
4-溴二苯甲酮 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-极淡的黄色
气味: 无资料
pH: 无数据资料
熔点:
82°C
沸点/沸程 176 °C/0.5kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
4-溴二苯甲酮 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 溴化氢
模块 11. 毒理学信息
急性毒性: ivn-mus LD50:100 mg/kg
ipr-rat LD50:100 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: DJ0350000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
4-溴二苯甲酮 修改号码:5
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4,4'-二溴二苯甲酮 bis(4-bromophenyl)methanone 3988-03-2 C13H8Br2O 340.014 —— (4-aminophenyl)(4-bromophenyl)methanone 40292-19-1 C13H10BrNO 276.132 二苯甲酮 benzophenone 119-61-9 C13H10O 182.222 4-溴二苯基甲烷 1-benzyl-4-bromo-benzene 2116-36-1 C13H11Br 247.134 4-氨基二苯甲酮 (4-aminophenyl)(phenyl)methanone 1137-41-3 C13H11NO 197.236 对溴苯甲醛 4-bromo-benzaldehyde 1122-91-4 C7H5BrO 185.02 1-(alpha-氯苄基)-4-溴苯 4-bromo-benzhydryl chloride 13391-38-3 C13H10BrCl 281.579 —— 1-bromo-4-(1-phenylethyl)benzene 56913-50-9 C14H13Br 261.161 对溴苯甲酰溴 1-bromo-4-(bromo(phenyl)methyl)benzene 18066-89-2 C13H10Br2 326.03 4-苄氧基苯甲酸 phenyl(4-bromophenyl)methanol 29334-16-5 C13H11BrO 263.134 1-溴-4-(1-苯基乙烯基)苯 1-(4-bromophenyl)-1-phenylethene 4333-76-0 C14H11Br 259.145 —— (4-bromophenyl)(phenyl)methanethione 1137-43-5 C13H9BrS 277.184 alpha-(4-溴苯基)苄胺 (4-bromophenyl)(phenyl)methanamine 55095-17-5 C13H12BrN 262.149 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (3-溴苯基)(4-溴苯基)甲酮 (3-bromophenyl)(4-bromophenyl)methanone 83699-51-8 C13H8Br2O 340.014 二苯甲酮 benzophenone 119-61-9 C13H10O 182.222 4-溴二苯基甲烷 1-benzyl-4-bromo-benzene 2116-36-1 C13H11Br 247.134 4-甲基二苯甲酮 4-Methylbenzophenone 134-84-9 C14H12O 196.249 4-苯甲酰基苯甲醛 4-benzoylbenzaldehyde 20912-50-9 C14H10O2 210.232 1,4-联苯酰基苯 1,4-dibenzoylbenzene 3016-97-5 C20H14O2 286.33 4-羟基-二苯甲酮 4-Hydroxybenzophenone 1137-42-4 C13H10O2 198.221 4-氨基二苯甲酮 (4-aminophenyl)(phenyl)methanone 1137-41-3 C13H11NO 197.236 (4-溴苯基)(2-氟-4-甲氧基苯基)甲酮 (4-bromophenyl)(2-hydroxyphenyl)methanone 2038-92-8 C13H9BrO2 277.117 2-氨基-4'-溴二苯甲酮 2-amino-4'-bromobenzophenone 1140-17-6 C13H10BrNO 276.132 4-碘二苯酮 4-iodobenzophenone 6136-66-9 C13H9IO 308.118 —— (4-mercaptophenyl)(phenyl)methanone 1620-94-6 C13H10OS 214.288 4-氟二苯甲酮 4-fluorobenzophenone 345-83-5 C13H9FO 200.212 —— [18F]-4-fluorobenzophenone 115216-92-7 C13H9FO 199.214 4-乙基苯甲酮 4-ethylbenzophenone 18220-90-1 C15H14O 210.276 —— (4-(fluoromethyl)phenyl)(phenyl)methanone 64747-74-6 C14H11FO 214.239 4-氰基苯甲酮 p-cyanobenzophenone 1503-49-7 C14H9NO 207.232 —— 4-vinylbenzophenone 3139-85-3 C15H12O 208.26 —— (4-ethynylphenyl)(phenyl)methanone 119754-17-5 C15H10O 206.244 1-(二苯基甲基)-4-溴苯 ((4-bromophenyl)methylene)dibenzene 5410-05-9 C19H15Br 323.232 —— (4-bromo-2-hydroxyphenyl)(phenyl)methanone 6723-04-2 C13H9BrO2 277.117 2-氨基-4’-溴二苯甲酮 (4-bromo-2-aminophenyl)phenyl-methanone 135776-98-6 C13H10BrNO 276.132 2-(4-苯甲酰基苯基)乙腈 2-(4-benzoylphenyl)acetonitrile 21192-61-0 C15H11NO 221.258 —— (4-(difluoromethyl)phenyl)(phenyl)methanone 64747-73-5 C14H10F2O 232.23 [4-(甲基氨基)苯基]-苯基甲酮 (4-(methylamino)phenyl)(phenyl)methanone 26178-74-5 C14H13NO 211.263 —— (4-allylphenyl)(phenyl)methanone 76385-38-1 C16H14O 222.287 4-异丙基二苯甲酮 4-isopropylbenzophenone 18864-76-1 C16H16O 224.302 4-甲氧基二苯甲酮 4-Methoxybenzophenone 611-94-9 C14H12O2 212.248 1-(alpha-氯苄基)-4-溴苯 4-bromo-benzhydryl chloride 13391-38-3 C13H10BrCl 281.579 1-(4-苯甲酰基苯基)乙酮 4-acetylbenzophenone 53689-84-2 C15H12O2 224.259 4-苯甲酰苯甲酸 4-carboxybenzophenone 611-95-0 C14H10O3 226.232 —— 4-benzoylphenyl methyl selenide 1272317-48-2 C14H12OSe 275.209 4-2-苯乙基苯甲酮 (4-phenethylphenyl)(phenyl)methanone 91036-10-1 C21H18O 286.373 —— 1-bromo-4-(1-phenylethyl)benzene 56913-50-9 C14H13Br 261.161 对溴苯甲酰溴 1-bromo-4-(bromo(phenyl)methyl)benzene 18066-89-2 C13H10Br2 326.03 4-苄氧基苯甲酸 phenyl(4-bromophenyl)methanol 29334-16-5 C13H11BrO 263.134 —— (S)-4-bromophenyl(phenyl)methanol 73773-07-6 C13H11BrO 263.134 —— (R)-(4-bromophenyl)(phenyl)methanol 2955-37-5 C13H11BrO 263.134 4-叔丁基苯甲酮 4-tert-butylbenzophenone 22679-54-5 C17H18O 238.329 (4’-甲基联苯-4-基)(苯基)甲酮 4-benzoyl-4'-methylbiphenyl 63283-56-7 C20H16O 272.346 4-苯基二苯甲酮 biphenyl-4-yl-phenyl-methanone 2128-93-0 C19H14O 258.32 —— 4,4'-dibenzoyl-1,1'-biphenyl 33090-29-8 C26H18O2 362.428 —— 4-methylthiobenzophenone 23405-48-3 C14H12OS 228.315 4-苄基苯甲醛 4-benzylbenzaldehyde 67468-65-9 C14H12O 196.249 1-溴-4-(1-苯基乙烯基)苯 1-(4-bromophenyl)-1-phenylethene 4333-76-0 C14H11Br 259.145 (4-丁基苯基)(苯基)甲酮 4-n-butylbenzophenone 55363-57-0 C17H18O 238.329 —— 4-benzoyldiphenylamine 4058-17-7 C19H15NO 273.334 —— phenyl(4-(p-tolylamino)phenyl)methanone 42872-23-1 C20H17NO 287.361 —— 4.4'-Dibenzoyl-diphenylamin 20953-62-2 C26H19NO2 377.442 苯基{4-[(三甲基硅烷基)甲基]苯基}甲酮 phenyl{4-[(trimethylsilyl)methyl]phenyl}methanone 1983-50-2 C17H20OSi 268.431 —— (4-bromophenyl)(phenyl)methanethione 1137-43-5 C13H9BrS 277.184 —— (4-(hydroxy(phenyl)methyl)phenyl)(phenyl)methanone 31020-20-9 C20H16O2 288.346 —— 3-(4-Benzoylphenyl)propanal 183230-81-1 C16H14O2 238.286 alpha-(4-溴苯基)苄胺 (4-bromophenyl)(phenyl)methanamine 55095-17-5 C13H12BrN 262.149 —— Phenyl[4-(2-phenylethenyl)phenyl]methanone 74685-66-8 C21H16O 284.357 苯基-[4-(2-苯基乙炔基)苯基]甲酮 4-(phenylethynyl)benzophenone 57542-59-3 C21H14O 282.342 —— bis(4-benzoylphenyl)acetylene 29673-44-7 C28H18O2 386.45 —— trans-4-Benzoylstilbene 20488-44-2 C21H16O 284.357 4-乙氧基二苯甲酮 4-ethoxybenzophenone 27982-06-5 C15H14O2 226.275 4-苯氧基苯甲酮 p-phenoxybenzophenone 6317-73-3 C19H14O2 274.319 —— bis-(4-benzoylphenyl) ether 6966-89-8 C26H18O3 378.427 4-苯甲酰苯硼酸 (4-benzoylphenyl)boronic acid 268218-94-6 C13H11BO3 226.04 - 1
- 2
- 3
- 4
- 5
- 6
- 7
反应信息
-
作为反应物:描述:4-溴苯甲酰苯 在 cesiumhydroxide monohydrate 、 (1,5-环辛二烯)二(三甲基硅烷基甲基)钯(II) 、 2-((di-adamantan-1-yl)phosphaneyl)-1-(2,6-diisopropylphenyl)-1H-imidazole 作用下, 以 四氢呋喃 为溶剂, 反应 20.0h, 以95%的产率得到4-羟基-二苯甲酮参考文献:名称:室温下钯催化芳基卤化物的羟基化摘要:降低热量:介绍了第一种室温下Pd催化的由芳基溴化物和氯化物合成苯酚的方法。使用笨重的咪唑基膦配体和新型钯前体[Pd(cod)(CH 2 SiMe 3)2 ]进行的钯介导的芳基卤化物羟基化反应的化学计量研究导致了在环境条件下高效催化合成苯酚的过程(参见方案) ; Ad =金刚烷基,cod = 1,5-环辛二烯)。DOI:10.1002/anie.200902148
-
作为产物:描述:1-溴-4-(苯基乙炔基)苯 在 氧气 、 copper diacetate 、 potassium carbonate 、 苯胺 作用下, 以 二甲基亚砜 为溶剂, 以58%的产率得到4-溴苯甲酰苯参考文献:名称:铜通过炔烃三价键的裂解从需氧炔烃中合成双芳基酮†摘要:据报道,一种新型的铜催化需氧化合物可通过C-C三键的裂解从1,2-二芳基炔烃中合成双芳基酮。该反应是1,2-二芳基炔烃向双芳基酮的新转化。DOI:10.1039/c4ra06460g
-
作为试剂:参考文献:名称:由与强还原性牺牲供体相关的均配铜(I)配合物光催化的脱卤反应摘要:为了以低成本和低毒性进行具有挑战性的光还原反应,我们的目标是首次使用具有简单、强烈着色的均质铜 ( I ) 配合物 [Cu(dipp) 2 ] + ( dipp = 2,9-二异丙基-1,10-菲咯啉)。该家族的配合物是弱光氧化剂,我们专门设计和合成了强大的、可回收的牺牲电子供体 D。我们证明,在 D 存在下用 LED 光照射期间,强还原剂 [Cu(dipp) 2 ] 0是有效地光生成。此外,我们展示了使用光生 [Cu(dipp) 2 ] 0的第一个光化学反应并且有证据表明整个反应的动力学受到牺牲供体E (D + /D)的氧化电位的强烈影响。因此,调整牺牲供体 D 和 [Cu(dipp) 2 ] +的热力学使我们能够开启一个全新的概念,从而获得廉价、无毒的太阳能光产生的非常强的还原能力。DOI:10.1039/d1cy01209f
文献信息
-
Ru(ii)-catalyzed intermolecular ortho-C–H amidation of aromatic ketones with sulfonyl azides作者:M. Bhanuchandra、M. Ramu Yadav、Raja K. Rit、Malleswara Rao Kuram、Akhila K. SahooDOI:10.1039/c3cc41915k日期:——Ru(II)-catalyzed intermolecular ortho-CâH amidation of weakly coordinating aromatic ketones with sulfonyl azides is reported. The developed reaction protocol can be extended to various substituted aromatic ketones to afford a wide range of desired CâN bond formation products in good yields.
-
Method For Decarboxylating C-C Cross-Linking Of Carboxylic Acids With Carbon Electrophiles申请人:Goossen Lukas公开号:US20080177114A1公开(公告)日:2008-07-24The invention relates to a method for decarboxylating C—C bond formation by reacting carboxylic salts with carbon electrophiles in the presence of transition metal compounds as catalysts. The method represents a decarboxylating C—C bond formation of carboxylic acid salts with carbon electrophiles, wherein the catalyst contains two transition metals and/or transition metal compounds, from which one is present, preferably, in the oxidation step, which are different from each other by one unit, and catalyzes a radical decarboxylation which is absorbed during the second oxidation steps, which are different from each other by two units and catalyzes the two electron processes of a C—C bond formation reaction.
-
Design and synthesis of an activity-based protein profiling probe derived from cinnamic hydroxamic acid作者:Teng Ai、Li Qiu、Jiashu Xie、Robert J. Geraghty、Liqiang ChenDOI:10.1016/j.bmc.2015.12.035日期:2016.2agents, we validated the anti-replicon activity of compound 1, a potent and selective anti-HCV hydroxamic acid recently reported by us. Generally favorable physicochemical and in vitro absorption, distribution, metabolism, and excretion (ADME) properties exhibited by 1 made it an ideal parent compound from which activity-based protein profiling (ABPP) probe 3 was designed and synthesized. Evaluation
-
Pd-Catalyzed Vinylation of Aryl Halides with Inexpensive Organosilicon Reagents Under Mild Conditions作者:Chu-Ting Yang、Jun Han、Jun Liu、Yi Li、Fan Zhang、Hai-Zhu Yu、Sheng Hu、Xiaolin WangDOI:10.1002/chem.201802573日期:2018.7.20Pd‐catalyzed Hiyama vinylation reaction of non‐activated aryl chlorides and bromides under mild conditions was developed. The use of efficient vinyl donors and electron‐rich sterically hindered phosphine ligands was critical for the success of the reaction. The products of this transformation can be used for Am/Cm separation, an important challenge in nuclear fuel reprocessing. The substituent effect
-
Efficient one-pot transformation of aminoarenes to haloarenes using halodimethylisulfonium halides generated in situ作者:Woonphil Baik、Wanqiang Luan、Hyun Joo Lee、Cheol Hun Yoon、Sangho Koo、Byeong Hyo KimDOI:10.1139/v05-026日期:2005.3.1
Halodimethylsulfonium halide 1, which is readily formed in situ from hydrohaloic acid and DMSO, is a good nucleophilic halide. This activated nucleophilic halide rapidly converts aryldiazonium salt prepared in situ by the same hydrohaloic acid and nitrite ion to aryl chlorides, bromides, or iodides in good yield. The combined action of nitrite ion and hydrohaloic acid in DMSO is required for the direct transformation of aromatic amines, which results in the production of aryl halides within 1 h. Substituted compounds with electron-donating or -withdrawing groups or sterically hindered aromatic amines are also smoothly transformed to the corresponding aromatic halides. The only observed by-product is the deaminated arene (usually <7%). The isolated aryldiazonium salts can also be converted to the corresponding aryl halides using 1. The present method offers a facile, one-step procedure for transforming aminoarenes to haloarenes and lacks the environmental pollutants that usually accompany the Sandmeyer reaction using copper halides. Key words: aminoarenes, haloarenes, halodimethylsulfonium halide, halogenation, amination.
卤二甲基亚砜卤化物1是一种良好的亲核卤化物,可在现场由氢卤酸和二甲亚砜形成。这种活化的亲核卤化物迅速将由相同的氢卤酸和亚硝酸根在现场制备的芳基重氮盐转化为芳基氯化物、溴化物或碘化物,收率较高。在DMSO中,亚硝酸根和氢卤酸的联合作用是直接转化芳香胺的必要条件,从而在1小时内产生芳基卤化物。带有电子给体或吸引基团或有立体位阻的芳香胺的取代化合物也可顺利转化为相应的芳香卤化物。观察到的唯一副产物是去氨基芳烃(通常<7%)。孤立的芳基重氮盐也可以使用1转化为相应的芳基卤化物。该方法提供了一种简便的、一步法的程序,用于将氨基芳烃转化为卤代芳烃,并且不伴随通常伴随使用铜卤化物进行桑迈尔反应的环境污染物。关键词:氨基芳烃,卤代芳烃,卤二甲基亚砜卤化物,卤化,胺化。
表征谱图
-
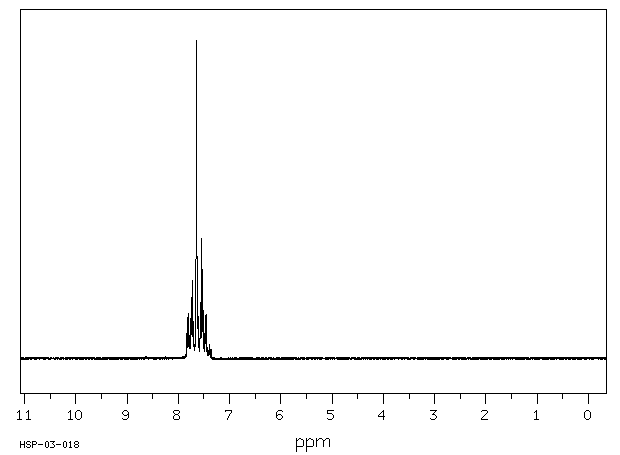
氢谱1HNMR
-
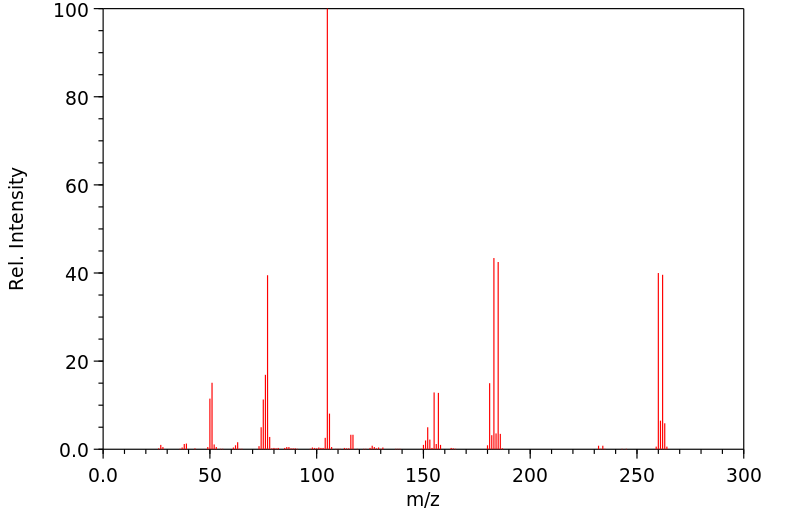
质谱MS
-
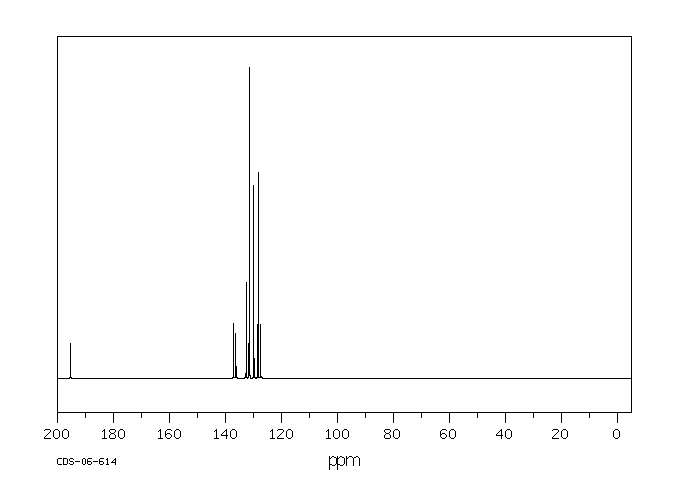
碳谱13CNMR
-
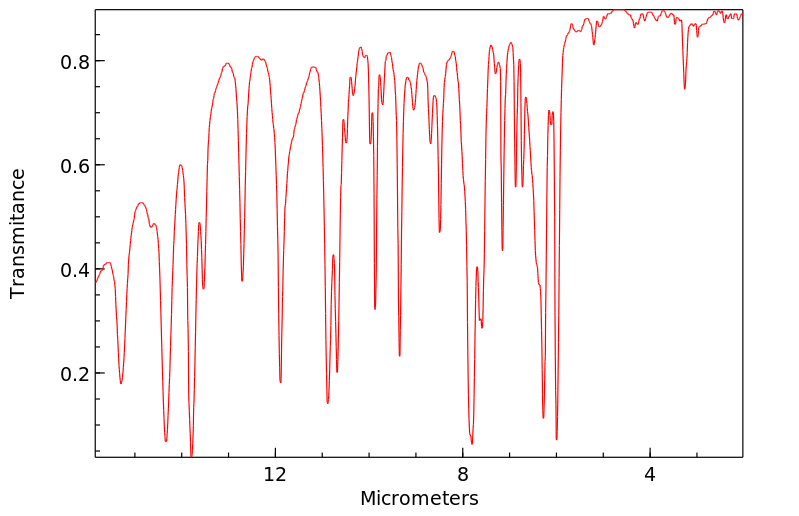
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息