L-谷氨酰胺 | 56-85-9
中文名称
L-谷氨酰胺
中文别名
L-2-氨基戊二酸酰胺;左谷酰胺;L-麸氨酰胺;(S)-2,5-二氨基-5-氧代戊酸;L-(+)-谷氨酰胺/2-氨基戊二酸;L-氨羰基丁氨酸;L-谷氨酸-5-酰胺;L-谷胺酰胺;L-谷酰胺;L-氨酰胺;L-谷氨酸酰胺;L-(+)-谷氨酰胺
英文名称
L-glutamine
英文别名
glutamine;Gln;L-Gln;L-glu;(2S)-5-amino-2-ammonio-5-oxopentanoate;(2S)-5-amino-2-azaniumyl-5-oxopentanoate
CAS
56-85-9
化学式
C5H10N2O3
mdl
MFCD00008044
分子量
146.146
InChiKey
ZDXPYRJPNDTMRX-VKHMYHEASA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:185 °C (dec.) (lit.)
-
比旋光度:32.25 º (c=10, 2 N HCl)
-
沸点:265.74°C (rough estimate)
-
密度:1.47 g/cm3 (20℃)
-
闪点:185°C
-
溶解度:在水中的溶解度25 mg/mL
-
最大波长(λmax):λ: 260 nm Amax: 0.01λ: 280 nm Amax: 0.01
-
LogP:-1.67
-
物理描述:DryPowder
-
颜色/状态:White crystalline powder
-
蒸汽压力:1.9X10-8 mm Hg at 25 °C (est)
-
水溶性:-0.55
-
稳定性/保质期:
-
分解:When heated to decomposition it emits toxic fumes of /nitrogen oxides/
-
解离常数:pK1 = 2.17, pK2 = 9.13
-
碰撞截面:133.7 Ų [M+Na]+ [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)]
计算性质
-
辛醇/水分配系数(LogP):-3.1
-
重原子数:10
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:106
-
氢给体数:3
-
氢受体数:4
ADMET
代谢
Exogenous L-glutamine likely follows the same metabolic pathways as endogenous L-glutamine which is involved in the formation of glutamate, proteins, nucleotides, and amino acid sugars.
来源:DrugBank
代谢
谷氨酰胺在氮平衡和肠道底物供应中起着重要作用。有研究表明,谷氨酰胺通过涉及精氨酸的肠道-肾脏途径,通过器官间运输的瓜氨酸,是精氨酸的前体。然而,肠道谷氨酰胺代谢对人类内源性精氨酸合成的重要性尚未得到解决。本研究的目的是探讨肠道将谷氨酰胺转化为瓜氨酸的过程以及肝脏对内脏瓜氨酸代谢的影响。8名接受上消化道手术的患者接受了[2-(15)N]谷氨酰胺和[脲基-(13)C-(2)H(2)]瓜氨酸的连续静脉输注。采集动脉、门静脉和肝静脉血液,并测量门静脉和肝血流量。根据建立的方法计算器官特异性氨基酸的摄取(处理)、产生和净平衡,以及全身血浆出现率。肠道消耗谷氨酰胺的速率取决于谷氨酰胺的供应。大约13%的肠道摄取的谷氨酰胺转化为瓜氨酸。从数量上看,谷氨酰胺是肠道释放瓜氨酸的唯一重要前体。肝脏既消耗又产生谷氨酰胺和瓜氨酸,但两种氨基酸的净肝流量与零没有显著差异。血浆谷氨酰胺是80%的血浆瓜氨酸的前体,而血浆瓜氨酸反过来又是10%的血浆精氨酸的前体。总之,谷氨酰胺在人类肠道转化为瓜氨酸后,是合成精氨酸的重要前体。
Glutamine plays an important role in nitrogen homeostasis and intestinal substrate supply. It has been suggested that glutamine is a precursor for arginine through an intestinal-renal pathway involving inter-organ transport of citrulline. The importance of intestinal glutamine metabolism for endogenous arginine synthesis in humans, however, has remained unaddressed. The aim of this study was to investigate the intestinal conversion of glutamine to citrulline and the effect of the liver on splanchnic citrulline metabolism in humans. Eight patients undergoing upper gastrointestinal surgery received a primed continuous intravenous infusion of [2-(15)N]glutamine and [ureido-(13)C-(2)H(2)]citrulline. Arterial, portal venous and hepatic venous blood were sampled and portal and hepatic blood flows were measured. Organ specific amino acid uptake (disposal), production and net balance, as well as whole body rates of plasma appearance were calculated according to established methods. The intestines consumed glutamine at a rate that was dependent on glutamine supply. Approximately 13% of glutamine taken up by the intestines was converted to citrulline. Quantitatively glutamine was the only important precursor for intestinal citrulline release. Both glutamine and citrulline were consumed and produced by the liver, but net hepatic flux of both amino acids was not significantly different from zero. Plasma glutamine was the precursor of 80% of plasma citrulline and plasma citrulline in turn was the precursor of 10% of plasma arginine. In conclusion, glutamine is an important precursor for the synthesis of arginine after intestinal conversion to citrulline in humans.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Endogenous glutamine participates in various metabolic activities, including the formation of glutamate, and synthesis of proteins, nucleotides, and amino sugars. Exogenous glutamine is anticipated to undergo similar metabolism.
来源:Hazardous Substances Data Bank (HSDB)
代谢
肠上皮细胞, 肝脏的
Enterocytes, Hepatic
来源:Toxin and Toxin Target Database (T3DB)
毒理性
辅助性L-谷氨酰胺的可能免疫调节作用可以通过多种方式来解释。L-谷氨酰胺似乎在保护胃肠道的完整性方面发挥着主要作用,尤其是大肠。在分解代谢状态下,肠粘膜的完整性可能会受损,从而导致肠道通透性增加,以及大肠中的革兰氏阴性细菌转移到体内。肠道对L-谷氨酰胺的需求,以及像淋巴细胞这样的细胞对L-谷氨酰胺的需求,似乎比骨骼肌提供的要大得多,骨骼肌是L-谷氨酰胺的主要储存组织。L-谷氨酰胺是肠上皮细胞、结肠细胞和淋巴细胞的优先呼吸燃料。因此,在这些条件下补充L-谷氨酰胺可能起到多种作用。首先,它可能通过节约骨骼肌中的L-谷氨酰胺来逆转分解代谢状态。它还可能抑制革兰氏阴性细菌从大肠转移。L-谷氨酰胺有助于维持分泌型IgA,其主要功能是防止细菌附着在粘膜细胞上。L-谷氨酰胺似乎对支持丝裂原刺激淋巴细胞的增殖以及白细胞介素-2(IL-2)和干扰素-γ(IFN-γ)的产生是必需的。它也是维持淋巴因子激活杀伤细胞(LAK)所必需的。L-谷氨酰胺可以增强中性粒细胞和单核细胞的吞噬作用。它可以导致肠道中谷胱甘肽的合成增加,这可能在通过减轻氧化应激来维持肠粘膜的完整性方面发挥作用。然而,补充L-谷氨酰胺可能的免疫调节作用的确切机制仍然不清楚。可以想象,L-谷氨酰胺的主要作用发生在肠道水平。也许肠道L-谷氨酰胺直接作用于肠道相关淋巴组织,并通过该机制刺激整体免疫功能,而不超过腹腔床。
Supplemental L-glutamine's possible immunomodulatory role may be accounted for in a number of ways. L-glutamine appears to play a major role in protecting the integrity of the gastrointestinal tract and, in particular, the large intestine. During catabolic states, the integrity of the intestinal mucosa may be compromised with consequent increased intestinal permeability and translocation of Gram-negative bacteria from the large intestine into the body. The demand for L-glutamine by the intestine, as well as by cells such as lymphocytes, appears to be much greater than that supplied by skeletal muscle, the major storage tissue for L-glutamine. L-glutamine is the preferred respiratory fuel for enterocytes, colonocytes and lymphocytes. Therefore, supplying supplemental L-glutamine under these conditions may do a number of things. For one, it may reverse the catabolic state by sparing skeletal muscle L-glutamine. It also may inhibit translocation of Gram-negative bacteria from the large intestine. L-glutamine helps maintain secretory IgA, which functions primarily by preventing the attachment of bacteria to mucosal cells. L-glutamine appears to be required to support the proliferation of mitogen-stimulated lymphocytes, as well as the production of interleukin-2 (IL-2) and interferon-gamma (IFN-gamma). It is also required for the maintenance of lymphokine-activated killer cells (LAK). L-glutamine can enhance phagocytosis by neutrophils and monocytes. It can lead to an increased synthesis of glutathione in the intestine, which may also play a role in maintaining the integrity of the intestinal mucosa by ameliorating oxidative stress. The exact mechanism of the possible immunomodulatory action of supplemental L-glutamine, however, remains unclear. It is conceivable that the major effect of L-glutamine occurs at the level of the intestine. Perhaps enteral L-glutamine acts directly on intestine-associated lymphoid tissue and stimulates overall immune function by that mechanism, without passing beyond the splanchnic bed.
来源:Toxin and Toxin Target Database (T3DB)
毒理性
在针对镰状细胞病患者的L-谷氨酰胺的临床试验中,没有提到血清转氨酶升高的情况,也没有报告出现临床上明显的肝损伤。镰状细胞病患者经常出现黄疸,这主要是由于慢性溶血导致血清间接胆红素水平升高。他们也可能因为镰状细胞病的并发症,如胆石病(由慢性溶血引起)、病毒性肝炎和铁过载(由输血引起)、充血性肝病(由于肺动脉高压)以及涉及肝脏的静脉闭塞危象,而出现肝功能测试异常波动,这些都可能导致血清转氨酶升高和肝功能障碍。在L-谷氨酰胺的预注册试验中,没有报告肝脏事件,而且与安慰剂相比,使用活性药物并未更常见严重不良事件。L-谷氨酰胺几乎是所有组织的正常组成部分,即使在较高剂量下也不太可能具有内在毒性。
谷氨酰胺的补充可能会加剧晚期肝硬化患者的肝性脑病。谷氨酰胺代谢为谷氨酸和氨,这可能会在严重肝功能障碍的患者中超过肝脏清除氨的能力。摄入10到20克的谷氨酰胺已被证明会导致血清氨水平升高,并恶化失代偿肝硬化患者的肝性脑病心理测量指标。在肝功能正常的患者中,谷氨酰胺补充不会增加血浆氨水平,其对肝硬化患者的影响并非由于肝损伤。然而,应避免在患有镰状细胞病和晚期肝硬化的患者中使用L-谷氨酰胺。
可能性评分:E(不太可能是导致急性肝损伤和黄疸的原因)。
In clinical trials of L-glutamine in patients with sickle cell disease, serum aminotransferase elevations were not mentioned, and there were no reports of clinically apparent liver injury. Patients with sickle cell disease frequently have jaundice, largely due to chronic hemolysis which raises serum indirect bilirubin levels. They also can have fluctuating liver test abnormalities due to complications of sickle cell disease, such as gall stone disease (from chronic hemolysis), viral hepatitis and iron overload (from blood transfusions), congestive liver disease (due to pulmonary hypertension), and veno-occlusive crises involving the liver which can be associated with serum aminotransferase elevations and hepatic dysfunction. In preregistration trials of L-glutamine, hepatic events were not reported and serious adverse events were no more common with the active drug than with placebo. L-glutamine is a normal constituent of virtually all tissues and is unlikely to have intrinsic toxicity, even in high doses.
Glutamine supplementation has a potential of worsening hepatic encephalopathy in patients with advanced cirrhosis. Glutamine is metabolized to glutamate and ammonia which can overwhelm the hepatic elimination of ammonia in patients with severe liver dysfunction. Ingestion of 10 to 20 grams of glutamine has been shown to cause elevations of serum ammonia levels and to worsen psychometric measures of hepatic encephalopathy in patients with decompensated cirrhosis. Plasma ammonia levels do not increase with glutamine supplementation in patients with normal hepatic function, and its effects in patients with cirrhosis is not due to hepatic injury. Nevertheless, use of L-glutamine should be avoided in patients with sickle cell disease and advanced cirrhosis.
Likelihood score: E (unlikely cause of acute liver injury with jaundice).
来源:LiverTox
毒理性
DILI标注:模糊的DILI关注
DILI Annotation:Ambiguous DILI-concern
来源:Drug Induced Liver Injury Rank (DILIrank) Dataset
毒理性
严重性等级:3
Severity Grade:3
来源:Drug Induced Liver Injury Rank (DILIrank) Dataset
吸收、分配和排泄
吸收效率高,通过主动运输机制发生。单次剂量后,Tmax为30分钟。多次剂量后的吸收动力学尚未确定。
Absorption is efficient and occurs by an active transport mechanism. Tmax is 30 minutes after a single dose. Absorption kinetics following multiple doses has not yet been determined.
来源:DrugBank
吸收、分配和排泄
主要通过代谢消除。尽管L-谷氨酰胺被滤过肾小球,但几乎全部被肾小管重吸收。
Primarily eliminated by metabolism. While L-glutamine is filtered though the glomerulus, nearly all is reabsorbed by renal tubules.
来源:DrugBank
吸收、分配和排泄
静脉推注后的分布体积为每公斤200毫升。
Volume of distribution is 200 mL/kg after intravenous bolus dose.
来源:DrugBank
吸收、分配和排泄
在三个受试者静脉注射 bolus 剂量后,估计分布容积大约为每公斤体重 200 毫升。
After an intravenous bolus dose in three subjects, the volume of distribution was estimated to be approximately 200 mL/kg.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
在六名受试者单次口服给予0.1克/公斤的谷氨酰胺后,平均血药峰浓度达到1028微摩尔(或150微克/毫升),大约在给药后30分钟出现。多次口服给药后的药代动力学特征尚未充分描述。
Following single dose oral administration of glutamine at 0.1 g/kg to six subjects, mean peak blood glutamine concentration was 1028uM (or 150 mcg/mL) occurring approximately 30 minutes after administration. The pharmacokinetics following multiple oral doses have not been adequately characterized.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S24/25
-
危险类别码:R36
-
WGK Germany:1,2
-
海关编码:2932999099
-
危险品运输编号:NONH for all modes of transport
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:本品应密封、阴凉处保存,并避免光照。
SDS
模块 1. 化学品
1.1 产品标识符
: L-谷氨酰胺 溶液
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.2 混合物
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
避免吸入蒸气、烟雾或气体。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
无数据资料
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
建议的贮存温度: -20 °C
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
飞溅保护
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
不需要对呼吸系统保护.对少量挥发请采用美国OV/AG (US)标准类型的 或欧洲ABEK (EU EN
14387)标准类型的呼吸器过滤器.
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 液体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
L-谷氨酰胺
非必需氨基酸 L-谷氨酰胺是蛋白质合成中的编码氨基酸之一,属于二十种非必需氨基酸。在人体内,它可以由谷氨酸、缬氨酸、异亮氨酸合成,也可以通过葡萄糖转化而来。
L-谷氨酰胺参与嘌呤和嘧啶核苷酸、氨基糖类、谷胱甘肽、L-谷氨酸及其他氨基酸的形成,并参与蛋白质合成与葡萄糖产生。与其他大多数氨基酸不同的是,在溶液中不稳定,降解速率受时间、温度及pH值的影响。
性状 L-谷氨酰胺又称麸氨酰胺或L-谷氨酸-5-酰胺,为白色针状结晶或粉末,分子量146.15。熔点约为184~185℃(分解)。溶于水而不溶于甲醇、乙醇、醚、苯、丙酮及氯仿、乙酸乙酯等有机溶剂,在中性溶液中稳定。在酸碱或热水中会分解成谷氨酸,或内酯化为吡咯羧酸,无臭且有微甜香味。本品在体内可转变为己糖胺,作为合成粘蛋白的前体,促进溃疡愈合。
鉴别试验 取0.1%试样液5ml,加水合茚三酮试液1ml,在沸水浴中加热溶解后,应呈紫红色。
含量分析 精确称取预经80℃干燥3h后的L-谷氨酰胺约150mg,溶于3ml甲酸和50ml冰醋酸后,用0.1mol/L高氯酸滴定,并以电位计测定终点。同时进行空白试验并作必要修正。每毫升0.1mol/L高氯酸相当于L-谷氨酰胺(C5H10NO4)293.16mg。
用途 广泛应用于生化研究、医药领域(治疗消化性溃疡、精神障碍、酒精中毒及癫痫病人脑功能障碍等疾病,改善智力发育不良儿童的状况)、营养增补剂与调味增香剂。此外,还用作脑功能改善剂和酒精中毒的治疗方法。
生产方法
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 DL-谷氨酰胺 GLUTAMIN 6899-04-3 C5H10N2O3 146.146 L-谷氨酸-酰胺-15N (5-15N)-L-Glutamine 59681-32-2 C5H10N2O3 147.139 D-谷氨酰胺 D-Glutamin 5959-95-5 C5H10N2O3 146.146 L-谷氨酸=gamma-肼 glutamic acid γ-hydrazide 1820-73-1 C5H11N3O3 161.161 茶氨酸 N-ethyl-L-glutamine 3081-61-6 C7H14N2O3 174.2 L-谷氨酸 L-glutamic acid 56-86-0 C5H9NO4 147.131 D-谷氨酸 D-Glu-OH 6893-26-1 C5H9NO4 147.131 L-正缬氨酸 L-Norvaline 6600-40-4 C5H11NO2 117.148 L-精氨酸 L-arginine 74-79-3 C6H14N4O2 174.203 L-正亮氨酸 L-Norleucine 327-57-1 C6H13NO2 131.175 L-谷氨酸-5-甲酯 H-L-Glu(OMe)-OH 1499-55-4 C6H11NO4 161.158 —— (S)-2-amino-4-cyano-butyric acid 6232-22-0 C5H8N2O2 128.131 L-赖氨酸 L-lysine 56-87-1 C6H14N2O2 146.189 (R)-2-氨基庚酸 D-2-aminoheptanoate 44902-01-4 C7H15NO2 145.202 (R)-2-氨基辛酸 D-2-aminooctanoate 106819-03-8 C8H17NO2 159.228 DL-2-氨基辛酸 2-aminooctanoic acid 644-90-6 C8H17NO2 159.228 乙酰谷酰胺 N-acetyl-L-glutamine 2490-97-3 C7H12N2O4 188.183 —— L-glutamine phosphate —— C5H11N2O6P 226.126 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 DL-谷氨酰胺 GLUTAMIN 6899-04-3 C5H10N2O3 146.146 D-谷氨酰胺 D-Glutamin 5959-95-5 C5H10N2O3 146.146 —— N-(γ-glutamyl)methylamine 3081-62-7 C6H12N2O3 160.173 L-谷氨酸gamma-单羟肟酸 L-Glutamic acid gamma monohydroxamate 1955-67-5 C5H10N2O4 162.145 L-谷氨酰胺甲酯 L-glutamine methyl ester 40846-98-8 C6H12N2O3 160.173 茶氨酸 N-ethyl-L-glutamine 3081-61-6 C7H14N2O3 174.2 —— N5-isopropyl-L-glutamine 4311-12-0 C8H16N2O3 188.227 L-谷氨酸 L-glutamic acid 56-86-0 C5H9NO4 147.131 DL-谷氨酸 Glutamic acid 617-65-2 C5H9NO4 147.131 —— N2-formyl-L-glutamine 91862-26-9 C6H10N2O4 174.156 —— N,N-dimethylglutamine —— C7H14N2O3 174.2 —— threo-β-Hydroxy-L-glutamine 110612-01-6 C5H10N2O4 162.145 —— azido-Gln 333366-22-6 C5H8N4O3 172.144 —— Glutamineon 59867-92-4 C5H10N2O2 130.147 gamma-L-谷氨酰-甘氨酸 (S)-2-Amino-4-(carboxymethyl-carbamoyl)-butyric acid 1948-29-4 C7H12N2O5 204.183 EPSILON-(GAMMA-L-谷氨酰)-L-赖氨酸 epsilon-(gamma-glutamyl)lysine 17105-15-6 C11H21N3O5 275.305 —— N-dodecanoyl glutamine —— C17H32N2O4 328.452 —— hexadecyl L-glutaminate 929697-36-9 C21H42N2O3 370.576 —— L-glutamine t-butyl ester —— C9H18N2O3 202.254 —— (S)-2-amino-4-cyano-butyric acid 6232-22-0 C5H8N2O2 128.131 L-赖氨酸 L-lysine 56-87-1 C6H14N2O2 146.189 乙酰谷酰胺 N-acetyl-L-glutamine 2490-97-3 C7H12N2O4 188.183 —— L-glutamine phosphate —— C5H11N2O6P 226.126 γ-L-谷氨酰-L-丙氨酸 H-γ-L-Glu-L-Ala-OH 5875-41-2 C8H14N2O5 218.21 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:Bellakki; Mahesh; Nandibewoor, Indian Journal of Chemistry, Section A: Inorganic, Physical, Theoretical and Analytical, 2004, vol. 43, # 8, p. 1639 - 1644摘要:DOI:
-
作为产物:描述:monosodium L-glutamate 在 imidazole buffer Pseudomonas taetrolens Y-30 glutamine synthetase EC 6.3.1.2 、 ammonium chloride 、 magnesium chloride 作用下, 生成 L-谷氨酰胺参考文献:名称:Purification and Characterization of Glutamine Synthetase ofPseudomonas taetrolensY-30: An Enzyme Usable for Production of Theanine by Coupling with the Alcoholic Fermentation System of Baker’s Yeast摘要:在含有面包酵母酒精发酵系统的混合物中,作为甲胺同化生物分离出来的假单胞菌Y-30的浓缩细胞提取物从谷氨酸和乙胺中生成了γ-谷氨酰乙酰胺(茶氨酸),用于ATP再生。在含有 1%甲胺、1%甘油、0.5%酵母提取物和 0.2%多肽作为碳源和氮源的培养基上生长的生物体的提取物中分离出了谷氨酰胺合成酶(GS),该酶可能负责形成茶氨酸。通过凝胶过滤估计其分子质量为 660 kDa,通过 SDS 聚丙烯酰胺凝胶电泳估计其分子质量为 55 kDa。该酶的活性需要 Mg2+ 或 Mn2+。在谷氨酰胺形成的标准反应条件下(pH 8.0,30 mM Mg2+),GS 对甲胺和乙胺的反应活性分别为对氨反应活性的 7% 和 1%。与烷基胺的反应活性随混合物中二价阳离子的最佳反应 pH 值的变化而变化:与乙胺的 Mg2+ 依赖性反应的最佳反应 pH 值为 11.0,而 Mn2+ 依赖性反应的最佳反应 pH 值为 8.5。在与 1000 毫摩尔乙胺(pH 值为 8.5 时含 3 毫摩尔 Mn2+)的最佳反应条件的混合物中,反应活性比标准反应条件下与氨的反应活性提高了 7%。分离出的 GS 在与酵母发酵系统的混合物中形成了茶氨酸。DOI:10.1271/bbb.68.1888
-
作为试剂:参考文献:名称:双重母体药物具有比母体螯合剂HBED更高的膜通透性,可提供出色的抗过氧化氢细胞保护作用摘要:N,N'-双(2-羟基苄基)乙二胺-N,N'-二乙酸(HBED)的临床使用因缺乏生物利用度而受到阻碍。N,N'-双(2-硼酸频哪醇酯苄基)乙二胺-N,N'-二乙酸甲酯,乙酯,异丙酯7 a – c及其二甲磺酸盐8 a – c是双重前药,它们分别掩盖了HBED的两个酚盐和两个羧酸盐供体,分别是硼酸酯和羧酸酯。在这里已经研究了它们通过化学水解和氧化作用的活化,它们的被动扩散性以及它们的细胞保护能力。8 a – c在37°C的最低基本介质中水解,半衰期分别为0.69、0.81和2.28 h。形成的中间体9 [ N,N'-双(2-硼酸苄基)乙二胺-N,N'-二乙酸],然后通过H 2 O 2进行氧化脱硼,得到HBED(k = 1.82 m-1 分钟-1)。在矿物油和磷酸盐缓冲液中的溶解度测量结果表明,与HBED相比,7 a在脂质和水溶解度之间具有更好的平衡。7a也能够被动扩散到类脂质硅膜上(对数通量=DOI:10.1002/cmdc.201600197
文献信息
-
<i>α-N</i>-Protected dipeptide acids: a simple and efficient synthesis via the easily accessible mixed anhydride method using free amino acids in DMSO and tetrabutylammonium hydroxide作者:G. Verardo、A. GorassiniDOI:10.1002/psc.2503日期:2013.5to find simple and efficient methods for their synthesis. For this reason, we have investigated the synthesis of α‐N‐protected dipeptide acids by reacting the easily accessible mixed anhydride of α‐N‐protected amino acids with free amino acids under different reaction conditions. The combination of TBA‐OH and DMSO has been found to be the best to overcome the low solubility of amino acids in organic在医学和药理学领域中,二肽的重要性已得到充分证明,并且已进行了许多努力来寻找简单有效的合成方法。因此,我们通过使易于获得的α-N混合酸酐反应,研究了α-N保护的二肽酸的合成在不同的反应条件下用游离氨基酸保护氨基酸。已经发现,TBA-OH和DMSO的组合是克服氨基酸在有机溶剂中低溶解度的最佳方法。在这些实验条件下,均相缩合反应迅速发生且没有可检测的差向异构。本方法也适用于未保护的侧链Tyr,Trp,Glu和Asp,但不适用于Lys。后一个残基能够结合两个混合酸酐分子,得到相应的异三肽。此外,已经测试了该方案对三肽和四肽合成的适用性。这种方法减少了对保护基的需求,具有成本效益,可扩展性,并产生了可用作较大肽合成的基础材料的二肽酸。
-
Anionic chiral cobalt(III) complexes as catalysts of asymmetric synthesis of cyanohydrins作者:Yu. N. Belokon’、V. I. Maleev、I. L. Mal’fanov、T. F. Savel’eva、N. S. Ikonnikov、A. G. Bulychev、D. L. Usanov、D. A. Kataev、M. NorthDOI:10.1007/s11172-006-0338-4日期:2006.5Chiral coordinatively saturated cobalt(III) complexes with Schiff bases of enantio-pure amino acids are formed as Λ and Δ-isomers, which are not transformed into each other under normal conditions. These complexes catalyze the formation of enantiomerically enriched cyanohydrins from aldehydes and Me3SiCN under homo-and heterogeneous catalysis.
-
IRAK DEGRADERS AND USES THEREOF申请人:Kymera Therapeutics, Inc.公开号:US20190192668A1公开(公告)日:2019-06-27The present invention provides compounds, compositions thereof, and methods of using the same.本发明提供了化合物、其组合物以及使用这些化合物的方法。
-
Microbial enantioselective removal of the N-benzyloxycarbonyl amino protecting group作者:Michèle Maurs、Francine Acher、Robert AzeradDOI:10.1016/j.molcatb.2012.03.005日期:2012.12Cbz-l-Glu as sole nitrogen source. A lyophilized whole-cell preparation of two Arthrobacter sp. strains grown on Cbz-Glu or Cbz-Gly exhibited a high cleavage activity. The conditions of hydrolysis have been optimized and a quantitative enantioselective deprotection of several Cbz-dl-amino acids was obtained, as well as the deprotection of N-carbamoylester derivatives of several synthetic amino compounds
-
Potentiometric and Speciation Studies on the Complex Formation Reactions of [Pd(2-methylaminomethyl)-pyridine)(H2O)2]2+ with Some Bio-active Ligands and Displacement Reaction of Coordinated Inosine作者:Abeer T. Abd El-Karim、Islam R. El-Sherif、Wafaa M. Hosny、Eyad K. Alkhadhairi、Mutlaq S. Aljahdali、Ahmed A. El-SherifDOI:10.1007/s10953-017-0621-z日期:2017.5of the complexes formed between [Pd(MAMP)(H2O)2]2+ and various biologically relevant ligands containing different functional groups were investigated. The ligands used are amino acids, peptides and DNA constituents. The results show the formation of 1:1 complexes with amino acids and peptides and the corresponding deprotonated amide species. Structural effects of peptides on amide deprotonation were研究了 [Pd(MAMP)(H2O)2]2+ 与含有不同官能团的各种生物相关配体之间形成的复合物的化学计量和稳定性常数。使用的配体是氨基酸、肽和 DNA 成分。结果显示与氨基酸和肽以及相应的去质子化的酰胺种类形成 1:1 复合物。研究了肽对酰胺去质子化的结构影响。嘌呤和嘧啶碱基尿嘧啶、尿苷、胞嘧啶、肌苷、肌苷 5'-单磷酸 (5'-IMP) 和胸腺嘧啶形成 1:1 和 1:2 复合物。计算各种复杂物质的浓度分布作为 pH 值的函数。还报道了氯离子浓度对 CBDCA 与 Pd(MAMP)2+ 形成常数的影响。结果显示 CBDCA 的开环和 DNA 成分的单齿络合形成 [Pd(MAMP)(CBDCA-O)DNA],其中 (CBDCA-O) 表示由一个羧酸氧配位的环丁烷二羧酸盐。计算了 SMC 和/或甲硫氨酸置换作为典型 DNA 成分的配位肌苷的平衡常数。预计结果将有助于抗肿瘤剂的化学。优化复合
表征谱图
-
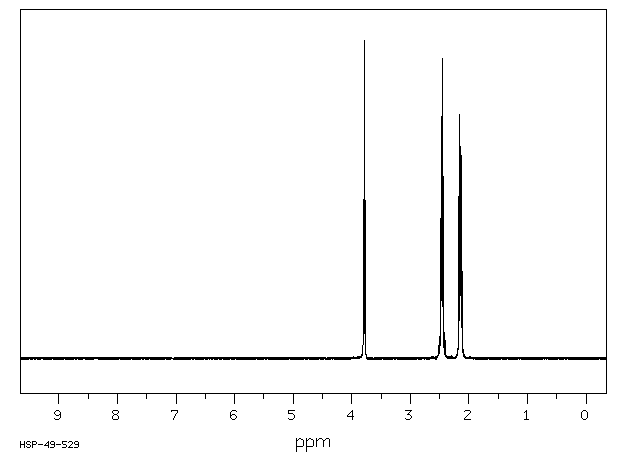
氢谱1HNMR
-
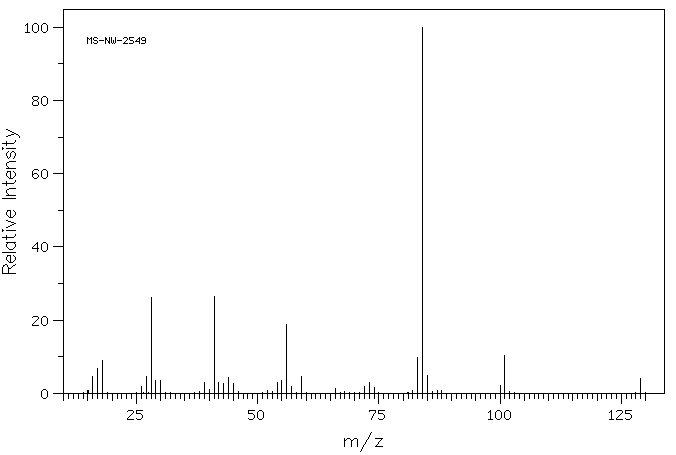
质谱MS
-
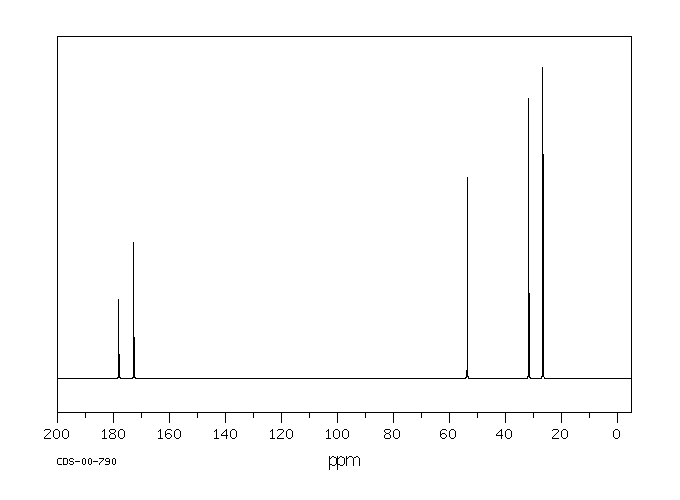
碳谱13CNMR
-
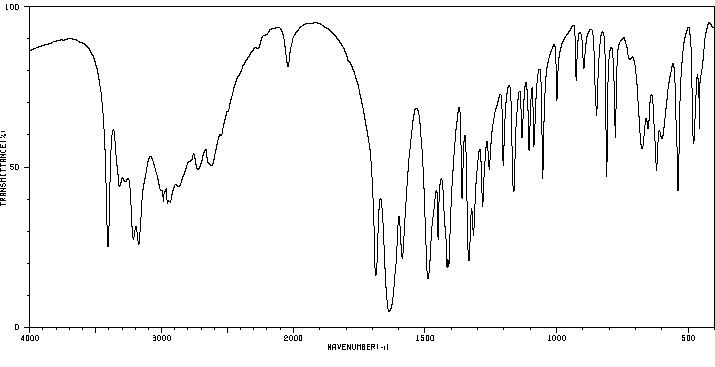
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸