4-异丙基苄氯 | 2051-18-5
中文名称
4-异丙基苄氯
中文别名
对异丙基氯苄;4-异丙基氯苄
英文名称
4-isopropylbenzyl chloride
英文别名
1-(chloromethyl)-4-propan-2-ylbenzene
CAS
2051-18-5
化学式
C10H13Cl
mdl
MFCD00018885
分子量
168.666
InChiKey
CYAKWEQUWJAHLW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:119 °C
-
密度:1.5230 g/mL at 25 °C(lit.)
-
闪点:206 °F
-
保留指数:1252
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,未有已知危险反应,应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:Yes
-
危险等级:8
-
危险品标志:Xi,C
-
危险类别码:R34,R36
-
危险品运输编号:UN 1760 8/PG 3
-
WGK Germany:3
-
海关编码:2903999090
-
包装等级:II
-
危险类别:8
-
安全说明:S26,S27,S36/37/39,S45
-
储存条件:请将贮藏器密封,并存放在阴凉、干燥处。确保工作环境有良好的通风或排气设施。
SDS
4-Isopropylbenzyl Chloride (contains ca. 10% o-form) Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 4-Isopropylbenzyl Chloride (contains ca. 10% o-form)
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 1
Corrosive to metals
HEALTH HAZARDS
Category 1B
Skin corrosion/irritation
Serious eye damage/eye irritation Category 1
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements May be corrosive to metals
Causes severe skin burns and eye damage
Precautionary statements:
[Prevention] Keep only in original container.
Do not breathe dust/fume/gas/mist/vapours/spray.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
Absorb spillage to prevent material damage.
[Storage] Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
form)
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 4-Isopropylbenzyl Chloride (contains ca. 10% o-form)
Percent: >85.0%(GC)
CAS Number: 2051-18-5
Synonyms: 7-Chloro-p-cymene
Chemical Formula: C10H13Cl
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Unsuitable extinguishing Solid streams of water
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use extra personal protective equipment (self-contained breathing apparatus). Keep
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Avoid contact with skin, eyes and clothing.
Advice on safe handling:
May develop pressure. Open carefully.
Use corrosive resistant equipment.
form)
Section 7. HANDLING AND STORAGE
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws. Keep only in original container.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust. Also install safety shower and eye bath.
Engineering controls:
Personal protective equipment
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Hand protection: Impervious gloves.
Safety goggles. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Liquid
Physical state (20°C):
Form: Clear
Colour: Colorless - Pale yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: No data available
104°C
Flash point:
Flammability or explosive
limits:
Lower: No data available
No data available
Upper:
Relative density: 1.02
Solubility(ies):
[Water] No data available
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen chloride
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
form)
Section 12. ECOLOGICAL INFORMATION
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
8: Corrosive.
Hazards Class:
UN-No: 3265
Proper shipping name: Corrosive liquid, acidic, organic, n.o.s.
Packing group: II
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 4-Isopropylbenzyl Chloride (contains ca. 10% o-form)
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 1
Corrosive to metals
HEALTH HAZARDS
Category 1B
Skin corrosion/irritation
Serious eye damage/eye irritation Category 1
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements May be corrosive to metals
Causes severe skin burns and eye damage
Precautionary statements:
[Prevention] Keep only in original container.
Do not breathe dust/fume/gas/mist/vapours/spray.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
Absorb spillage to prevent material damage.
[Storage] Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
form)
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 4-Isopropylbenzyl Chloride (contains ca. 10% o-form)
Percent: >85.0%(GC)
CAS Number: 2051-18-5
Synonyms: 7-Chloro-p-cymene
Chemical Formula: C10H13Cl
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Unsuitable extinguishing Solid streams of water
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use extra personal protective equipment (self-contained breathing apparatus). Keep
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Avoid contact with skin, eyes and clothing.
Advice on safe handling:
May develop pressure. Open carefully.
Use corrosive resistant equipment.
form)
Section 7. HANDLING AND STORAGE
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws. Keep only in original container.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust. Also install safety shower and eye bath.
Engineering controls:
Personal protective equipment
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Hand protection: Impervious gloves.
Safety goggles. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Liquid
Physical state (20°C):
Form: Clear
Colour: Colorless - Pale yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: No data available
104°C
Flash point:
Flammability or explosive
limits:
Lower: No data available
No data available
Upper:
Relative density: 1.02
Solubility(ies):
[Water] No data available
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen chloride
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
form)
Section 12. ECOLOGICAL INFORMATION
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
8: Corrosive.
Hazards Class:
UN-No: 3265
Proper shipping name: Corrosive liquid, acidic, organic, n.o.s.
Packing group: II
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-异丙基甲苯 4-methylisopropylbenzene 99-87-6 C10H14 134.221 异丙苯 Isopropylbenzene 98-82-8 C9H12 120.194 4-异丙基苯甲醛 (4-isopropylbenzaldehyde) 122-03-2 C10H12O 148.205 4-异丙基苯甲醇 cuminol 536-60-7 C10H14O 150.221 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-异丙基苯乙腈 (4-isopropylphenyl)acetonitrile 4395-87-3 C11H13N 159.231 1-异丙基-4-丙基苯 1-isopropyl-4-propylbenzene 22975-62-8 C12H18 162.275 4-异丙基苯甲醛 (4-isopropylbenzaldehyde) 122-03-2 C10H12O 148.205 4-异丙基苯甲醇 cuminol 536-60-7 C10H14O 150.221 4-异丙基苄胺 4-isopropylbenzylamine 4395-73-7 C10H15N 149.236 对异丙基溴苄 4-i-propylbenzyl bromide 73789-86-3 C10H13Br 213.117 —— 1,2-bis(4-isopropylphenyl)ethane 5789-33-3 C20H26 266.426 —— 1-isopropyl-4-neopentylbenzene 37920-33-5 C14H22 190.329 (4-异丙基-苄基)-甲胺 1-(4-isopropylphenyl)-N-methylmethanamine 73441-51-7 C11H17N 163.263 —— p-(methoxymethyl)-isopropylbenzene 73789-85-2 C11H16O 164.247 4-异丙基苯乙酮 1-(4-isopropylphenyl)propan-2-one 7306-39-0 C12H16O 176.258 —— 1-benzyl-4-isopropylbenzene 886-58-8 C16H18 210.319 4-异丙基苯乙酸 (4-isopropylphenyl)acetic acid 4476-28-2 C11H14O2 178.231 双(4-异丙基苄基)胺 bis(4-isopropylbenzyl)amine 202849-30-7 C20H27N 281.441 —— ethyl-(4-isopropyl-benzyl)-ether —— C12H18O 178.274 1-(环丙基甲基)-4-异丙基苯 1-cyclopropylmethyl-4-(1-methylethyl)benzene 401584-82-5 C13H18 174.286 4-环丙基苯甲醛 4-cyclopropylbenzaldehyde 20034-50-8 C10H10O 146.189 兔耳草醛 cyclamenaldehyde 103-95-7 C13H18O 190.285 —— dicymyl ether 146689-61-4 C20H26O 282.426 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:Oxidation of Benzyl Chlorides and Bromides to Benzoic Acids with 30 Hydrogen Peroxide in the Presence of Na2WO4, Na2VO4, or Na2MoO4 under Organic Solvent-Free Conditions摘要:DOI:10.1021/jo001796n
-
作为产物:参考文献:名称:van Zanten,B.; Nauta,W., Recueil des Travaux Chimiques des Pays-Bas, 1960, vol. 79, p. 1211 - 1222摘要:DOI:
-
作为试剂:描述:丙基溴化镁 、 7-甲氧基-3,7-二甲基-1-辛烯 在 Titanocene dichloride 4-异丙基苄氯 、 四氢呋喃 、 cuprous chloride 、 氯化铵 、 正戊烷 作用下, 以 四氢呋喃 为溶剂, -10.0~75.0 ℃ 、5.33 kPa 条件下, 反应 35.0h, 以to give product (2,6-dimethyl-2-methoxy-9-isopropylphenylnonane) (12.5 g; bp 150°-152° /0.25-0.3 torr)的产率得到2,6-Dimethyl-2-methoxy-9-isopropylphenylnonane参考文献:名称:Process for preparation of arylterpenoid insect maturation inhibitors摘要:提供了一种改进的生产生物活性芳基萜类化合物的方法,可用于抑制蛹的羽化,例如苍蝇蛹或蚊子蛹。该方法的特点是将具有末端不饱和键的萜类物质(例如双氢没食子醇(3,7-二甲基辛-1,6-二烯))与低级烷基格氏试剂(例如正丙基氯化镁)反应,形成格氏试剂交换产物。然后对交换产物进行苄基化。可以使用苄卤化物(例如对异丙基苄基氯化物)或苯甲醛(例如对异丙基苯甲醛)。这些化合物在美国专利4,002,769中有具体描述。它们还包含一个低级烷氧基团(例如甲氧基)。这可以在形成格氏试剂交换产物之前或操作的后期引入。使用交换型格氏反应可以消除从松脂素作为萜类来源生产芳基萜类化合物时的几个步骤,从而降低成本。公开号:US04484007A1
文献信息
-
A General, Activator-Free Palladium-Catalyzed Synthesis of Arylacetic and Benzoic Acids from Formic Acid作者:Lin Wang、Helfried Neumann、Matthias BellerDOI:10.1002/anie.201802384日期:2018.6.4carboxylative synthesis of arylacetic and benzoic acids using formic acid (HCOOH) as the CO surrogate was developed. In an improvement over previous work, CO is generated in situ without the need for any additional activators. Key to success was the use of a specific system consisting of palladium acetate and 1,2‐bis((tert‐butyl(2‐pyridinyl)phosphinyl)methyl)benzene. The generality of this method is demonstrated
-
N‐Heterocyclic Carbene (NHC)‐Stabilized Ru <sup>0</sup> Nanoparticles: In Situ Generation of an Efficient Transfer Hydrogenation Catalyst作者:Lakshay Kathuria、Noor U. Din Reshi、Ashoka G. SamuelsonDOI:10.1002/chem.202000142日期:2020.6.18untethered ruthenium half‐sandwich complexes were synthesized and characterized spectroscopically. X‐ray crystallographic analysis of three untethered and two tethered Ru N‐heterocyclic carbene (NHC) complexes were also carried out. These RuNHC complexes catalyze transfer hydrogenation of aromatic ketones in 2‐propanol under reflux, optimally in the presence of (25 mol %) KOH. Under these conditions, the束缚和未束缚的钌半三明治复合物通过光谱法表征。还进行了三个未束缚的和两个束缚的Ru N-杂环卡宾(NHC)配合物的X射线晶体学分析。这些RuNHC配合物可在回流条件下(2-5%的KOH)存在下,在2-丙醇中催化芳族酮的转移加氢反应。在这些条件下,形成2–3 nm大小的Ru 0通过TEM测量检测纳米颗粒。对纳米颗粒的固态NMR研究表明,NHC配体与Ru纳米颗粒(NPs)的表面结合。与以前已知的通向NHC稳定化的Ru纳米催化剂的途径相比,这种由碱促进的直接从芳族化的钌-NHC络合物和不受束缚的钌-NHC络合物到NHC稳定的钌纳米颗粒的途径更方便。[RuCl 2(p- cymene)] 2混合物的反应也产生了类似的催化活性RuNP。NHC前体与KOH在异丙醇中回流。这些经过NHC稳定的RuNP催化的转移氢化具有很高的周转率。如果纳米颗粒暴露于空气中或通过在反应期间冷却反应混合物而使其聚集和沉淀,则催化效率显着降低。
-
Catalytic Staudinger Reduction at Room Temperature作者:Danny C. Lenstra、Joris J. Wolf、Jasmin MecinovićDOI:10.1021/acs.joc.9b00831日期:2019.5.17catalytic Staudinger reduction at room temperature that enables the preparation of a structurally diverse set of amines from azides in excellent yields. The reaction is based on the use of catalytic amounts of triphenylphosphine as a phosphine source and diphenyldisiloxane as a reducing agent. Our catalytic Staudinger reduction exhibits a high chemoselectivity, as exemplified by reduction of azides
-
Oxidation of Benzyl Halides to Aldehydes and Ketones with Potassium Nitrate Catalyzed by Phase-Transfer Catalyst in Aqueous Media作者:Qifa Liu、Ming Lu、Feng Sun、Jiang Li、Yuebing ZhaoDOI:10.1080/00397910802323080日期:2008.11.3Abstract The catalytic oxidation of benzyl halides to aldehydes and ketones in aqueous media was studied under relatively mild reaction conditions by using phase-transfer catalyst combined with potassium nitrate and 10% aqueous potassium hydroxide solution. As a result, a simple high-yield procedure has been developed.
-
Substituted phenyl farnesyltransferase inhibitors申请人:——公开号:US20020019527A1公开(公告)日:2002-02-14Compounds of formula (I) 1 or pharmaceutically acceptable salts thereof, inhibit farnesyltransferase. Methods for making the compounds, pharmaceutical compositions containing the compounds, and methods of treatment using the compounds are disclosed.式(I)的化合物或其药学上可接受的盐,抑制法尼基转移酶。公开了制备这些化合物的方法,含有这些化合物的药物组合物,以及使用这些化合物进行治疗的方法。
表征谱图
-
氢谱1HNMR
-
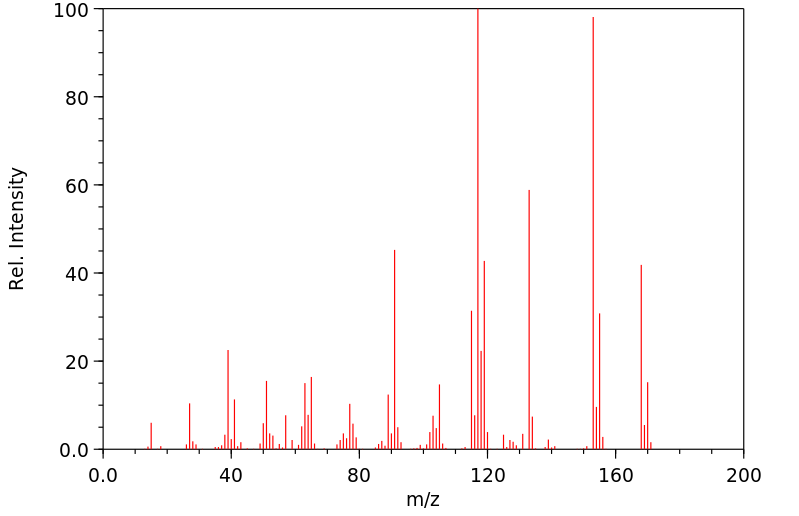
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸