N(omega)-甲基色胺 | 61-49-4
中文名称
N(omega)-甲基色胺
中文别名
N-甲基色胺;3-(2-甲基氨基乙基)吲哚;N-Ω-甲基色胺;N-OMEGA-甲基色胺
英文名称
N-Methyltryptamine
英文别名
[2-(1H-indol-3-yl)-ethyl]-methylamine;2-(1H-indol-3-yl)-N-methylethan-1-amine;3-(2-[methylamino]ethyl)indole;Nω-methyltryptamine;2-(1H-indol-3-yl)-N-methylethanamine
CAS
61-49-4
化学式
C11H14N2
mdl
——
分子量
174.246
InChiKey
NCIKQJBVUNUXLW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:87-89 °C(lit.)
-
沸点:295.23°C (rough estimate)
-
密度:0.9649 (rough estimate)
-
溶解度:DMF:11mg/mL; DMSO:5mg/mL;乙醇:20mg/mL;乙醇:PBS(pH 7.2)(1:1):0.5 mg/ml;甲醇:1mg/mL
-
物理描述:Solid
-
碰撞截面:142.5 Ų [M+H]+ [CCS Type: DT, Method: single field calibrated with ESI Low Concentration Tuning Mix (Agilent)]
-
保留指数:1770;1745
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:13
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.272
-
拓扑面积:27.8
-
氢给体数:2
-
氢受体数:1
安全信息
-
安全说明:S24/25
-
WGK Germany:3
-
RTECS号:NM0450000
-
海关编码:2933990090
-
储存条件:-20°C
SDS
| Name: | n-Omega-methyltryptamine 99% Material Safety Data Sheet |
| Synonym: | 3-(2-Methylaminoethyl)indole; 1H-Indole-3-ethanamine, N-methyl-; 3-(2-(Methylamino)ethyl)indole; Indole, 3-(2-(methylamino)ethyl)-; Methyltryptamine; N-Methyltryptamine; N-Monomethyltryptamin |
| CAS: | 61-49-4 |
Synonym:3-(2-Methylaminoethyl)indole; 1H-Indole-3-ethanamine, N-methyl-; 3-(2-(Methylamino)ethyl)indole; Indole, 3-(2-(methylamino)ethyl)-; Methyltryptamine; N-Methyltryptamine; N-Monomethyltryptamin
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 61-49-4 | N-omega-Methyltryptamine | 99 | 200-507-9 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 61-49-4: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 87.00 - 89.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Not available.
Specific Gravity/Density: Not available.
Molecular Formula: C11H14N2
Molecular Weight: 174.24
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, nitrogen.
Hazardous Polymerization: Will not occur.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 61-49-4: NM0450000 LD50/LC50:
Not available.
Carcinogenicity:
N-omega-Methyltryptamine - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 61-49-4: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 61-49-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 61-49-4 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-[2-(1H-吲哚-3-基)乙基]甲酰胺 Nb-formyltryptamine 6502-82-5 C11H12N2O 188.229 色胺 tryptamine 61-54-1 C10H12N2 160.219 —— methyl 2-(1-H-indol-3-yl)ethylcarbamate 58635-45-3 C12H14N2O2 218.255 —— ethyl 2-(1H-indole-3-yl)ethylcarbamate 67909-99-3 C13H16N2O2 232.282 [2-(1H-吲哚-3-基)乙基]氨基甲酸叔丁酯 tert-butyl [2-(1H-indol-3-yl)ethyl]carbamate 103549-24-2 C15H20N2O2 260.336 吲哚-3-乙腈 3-indoleacetonitrile 771-51-7 C10H8N2 156.187 3-(2-溴乙基)吲哚 3-(2-bromoethyl)-1H-indole 3389-21-7 C10H10BrN 224.1 色醇 2-(3-indole)-ethanol 526-55-6 C10H11NO 161.203 苄基(2-(1H-吲哚-3-基)乙基)氨基甲酸酯 (2-indol-3-yl-ethyl)-carbamic acid benzyl ester 38750-13-9 C18H18N2O2 294.353 相思豆毒素 L-abrine 526-31-8 C12H14N2O2 218.255 2-(1H-吲哚-3-基)-N-甲基-2-氧代乙酰胺 2-(1H-indol-3-yl)-N-methyl-2-oxoacetamide 2054-72-0 C11H10N2O2 202.213 —— N-(2-(1H-indol-3-yl)ethyl)-N,4-dimethylbenzenesulfonamide 442531-80-8 C18H20N2O2S 328.435 吲哚-3-乙醛酰氯 indolyl-3-glyoxylyl chloride 22980-09-2 C10H6ClNO2 207.616 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N,N-二甲基色胺 N,N-dimethyltryptamine 61-50-7 C12H16N2 188.272 —— N-methyl-N-formyltryptamine 54268-27-8 C12H14N2O 202.256 N-[2-(1H-吲哚-3-基)乙基]-N-甲基-2-丙胺 N-methyl-N-isopropyltryptamine 96096-52-5 C14H20N2 216.326 2-[(2-吲哚-3-基-乙基)-甲基-氨基]-乙醇 2-((2-(1H-indol-3-yl)ethyl)(methyl)amino)ethan-1-ol 101264-45-3 C13H18N2O 218.299 —— N-methyl-N-acetyltryptamine 91821-04-4 C13H16N2O 216.283 —— 1-[(2-indol-3-yl-ethyl)-methyl-amino]-propan-2-ol 108238-75-1 C14H20N2O 232.326 —— (2E,4E)-5-[2-(1H-indol-3-yl)ethyl-methylamino]penta-2,4-dienal 1352708-24-7 C16H18N2O 254.332 —— N-methoxycarbonyl-N-methyltryptamine 63637-70-7 C13H16N2O2 232.282 —— N-methyl-2-(6-chloro-1H-indol-3-yl)ethylamine —— C11H13ClN2 208.691 —— 2-butynoic acid [2-(1H-indol-3-yl)ethyl]methylamide 1179531-44-2 C15H16N2O 240.305 —— N-(2-indol-3-yl-ethyl)-N-methyl-2-phenyl-acetamide 19462-25-0 C19H20N2O 292.381 —— 2-{[2-(1H-Indol-3-yl)-ethyl]-methyl-amino}-1-phenyl-ethanone 760957-11-7 C19H20N2O 292.381 —— (4-ethynylbenzyl)-[2-(1H-indol-3-yl)-ethyl]methylamine 894360-62-4 C20H20N2 288.392 —— N10-BOC-N10-methyltryptamine 264619-90-1 C16H22N2O2 274.363 2-(5-甲氧基-1H-吲哚-3-基)-N-甲基乙胺 5-methoxy-N-methyltryptamine 2009-03-2 C12H16N2O 204.272 —— N-methyl-N-(4-carboxybutyryl)-tryptamine 75622-16-1 C16H20N2O3 288.346 —— 1-(4-bromophenyl)-2-[2-(1H-indol-3-yl)ethyl-methylamino]ethanone 760957-20-8 C19H19BrN2O 371.277 —— 1-(4-Chloro-phenyl)-2-{[2-(1H-indol-3-yl)-ethyl]-methyl-amino}-ethanone 760957-18-4 C19H19ClN2O 326.826 —— N-indol-3-ylmethyl-N-methyltryptamine 205384-48-1 C20H21N3 303.407 —— N-methyl-N-(4-methoxycarbonylbutyryl)-tryptamine 75622-04-7 C17H22N2O3 302.373 —— CA192 16531-06-9 C18H18N2O 278.354 —— CA233 —— C19H20N2O 292.381 —— CA223 —— C18H17ClN2O 312.799 —— CA238 883560-98-3 C18H17BrN2O 357.25 —— CA230 —— C19H20N2O 292.381 —— CA220 —— C18H17FN2O 296.344 —— 1-hydroxy-N-methoxycarbonyl-N-methyltryptamine 205384-46-9 C13H16N2O3 248.282 —— CA225 —— C22H26N2O 334.461 —— 5-methoxy-N-methoxycarbonyl-N-methyltryptamine 205384-47-0 C14H18N2O3 262.309 —— CA222 —— C18H17ClN2O 312.799 —— CA234 —— C18H17BrN2O 357.25 —— CA224 —— C24H22N2O 354.451 —— CA219 —— C18H17FN2O 296.344 —— CA229 —— C19H20N2O 292.381 —— CA221 —— C18H17ClN2O 312.799 —— S-(3-phenylpropyl) 4-[[2-(1H-indol-3-yl)ethyl-methylamino]methyl]benzenecarbothioate 1178916-65-8 C28H30N2OS 442.625 —— CA237 —— C18H17BrN2O 357.25 —— CA218 —— C18H17FN2O 296.344 —— N1-benzyl-N10-methyltryptamine 56999-38-3 C18H20N2 264.37 —— CA228 —— C19H20N2O2 308.38 —— 1-hydroxy-N-indol-3-ylmethyl-N-methyltryptamine 205384-50-5 C20H21N3O 319.406 —— N,N'-bis[2-(1H-indol-3-yl)-ethyl]-N,N'-dimethyl-phthalamide 521949-80-4 C30H30N4O2 478.594 —— Nb-formyl-Nb-methyl-1-(3-methylbut-2-enyl)tryptamine 80693-56-7 C17H22N2O 270.374 —— (2R)-2-benzyl-3-<<(2-indol-3-ylethyl)methylamino>carbonyl>propionic acid 146827-55-6 C22H24N2O3 364.444 —— N-(2-(1H-indol-3-yl)ethyl)-N,4-dimethylbenzenesulfonamide 442531-80-8 C18H20N2O2S 328.435 —— N-indol-3-ylmethyl-5-methoxy-N-methyltryptamine —— C21H23N3O 333.433 2-甲基-9H-1,2,3,4-四氢吡啶并(3,4-b)吲哚 1,2,3,4-tetrahydro-2-methyl-9H-pyrido[3,4-b]indole 13100-00-0 C12H14N2 186.257 —— Evodiamide 116965-70-9 C19H21N3O 307.4 —— CA226 —— C19H20N2O2 308.38 —— N-2-[2-(1,1-dimethylallyl)-1H-indol-3-yl]ethyl-N-methylformamide 863885-34-1 C17H22N2O 270.374 —— ethyl 2-[4-[[2-(1H-indol-3-yl)ethyl-methylamino]methyl]piperidin-1-yl]pyrimidine-5-carboxylate 875318-48-2 C24H31N5O2 421.542 —— N-(2-(1H-indol-3-yl)ethyl)-N-methyl-4-nitrobenzenesulfonamide 1280171-15-4 C17H17N3O4S 359.406 —— 3-(3-{[2-(1H-Indol-3-yl)-ethyl]-methyl-amino}-propyl)-7,8-dimethoxy-1,3,4,5-tetrahydro-benzo[d]azepin-2-one 98784-70-4 C26H33N3O3 435.566 - 1
- 2
- 3
- 4
- 5
- 6
反应信息
-
作为反应物:描述:参考文献:名称:抗真菌吲哚衍生物摘要:硝基甲烷与吲哚基硝基乙烯 2 的迈克尔加成生成 1,3-二硝基-2-(吲哚-3'-基)-丙烷 3. - 醛 4 和 10 与苄胺 12 和吲哚烷基胺 6a 和 9a 与苯甲醛 11 和席夫碱的还原导致 N-苄基-(吲哚-3-基甲基)-胺 13 和 N-苄基-(吲哚-3-基乙基)-胺 14。 - 叔胺 16 通过甲酰胺形成15,根据Mannich合成的胺18。- 检查所有化合物对结核分枝杆菌 H 37 Ra 的生长抑制作用。根据结构讨论抗真菌活性。DOI:10.1002/ardp.19943270209
-
作为产物:描述:相思豆毒素 320.0 ℃ 、1.6 kPa 条件下, 生成 N(omega)-甲基色胺参考文献:名称:Hoshino, Justus Liebigs Annalen der Chemie, 1935, vol. 520, p. 31,33摘要:DOI:
-
作为试剂:描述:benzofuran-6-carbonyl chloride 在 N(omega)-甲基色胺 、 盐酸-N-乙基-Nˊ-(3-二甲氨基丙基)碳二亚胺 、 N,N-二异丙基乙胺 作用下, 以 四氢呋喃 、 乙腈 为溶剂, 反应 3.0h, 生成 立他司特参考文献:名称:PROCESS FOR PREPARING LIFITEGRAST AND INTERMEDIATES THEREOF摘要:本公开提供了合成利非替格和其中间体的高效、经济、改进的过程。目前公开的过程提供了一条直接的合成路线,避免了保护或脱保护步骤。目前公开的过程还提供了使用较少合成步骤合成利非替格的方法。公开号:US20190002445A1
文献信息
-
[EN] BIS-HETEROARYL DERIVATIVES AS MODULATORS OF PROTEIN AGGREGATION<br/>[FR] DÉRIVÉS BIS-HÉTÉROARYLIQUES EN TANT QUE MODULATEURS DE L'AGRÉGATION DES PROTÉINES申请人:NEUROPORE THERAPIES INC公开号:WO2017020010A1公开(公告)日:2017-02-02The present invention relates to certain bis-heteroaryl compounds, pharmaceutical compositions containing them, and methods of using them, including methods for preventing, reversing, slowing, or inhibiting protein aggregation, and methods of treating diseases that are associated with protein aggregation, including neurodegenerative diseases such as Parkinson's disease, Alzheimer's disease, Lewy body disease, Parkinson's disease with dementia, fronto- temporal dementia, Huntington's Disease, amyotrophic lateral sclerosis, and multiple system atrophy, and cancer including melanoma.
-
[EN] LYMPHATIC SYSTEM-DIRECTING LIPID PRODRUGS<br/>[FR] PROMÉDICAMENTS LIPIDIQUES ORIENTANT VERS LE SYSTÈME LYMPHATIQUE申请人:ARIYA THERAPEUTICS INC公开号:WO2019046491A1公开(公告)日:2019-03-07The present invention provides lymphatic system-directing lipid prodrugs, pharmaceutical compositions thereof, methods of producing such prodrugs and compositions, as well as methods of improving the bioavailability or other properties of a therapeutic agent that comprises part of the lipid prodrug. The present invention also provides methods of treating a disease, disorder, or condition such as those disclosed herein, comprising administering to a patient in need thereof a provided lipid prodrug or a pharmaceutical composition thereof.本发明提供了淋巴系统定向脂质前药,其制药组合物,制备这种前药和组合物的方法,以及改善作为脂质前药一部分的治疗剂的生物利用度或其他性质的方法。本发明还提供了治疗疾病、紊乱或症状的方法,包括向需要的患者施用所提供的脂质前药或其制药组合物。
-
Aminopyrimidine Kinase Inhibitors申请人:Baldino Carmen M.公开号:US20110152235A1公开(公告)日:2011-06-23Disclosed are compounds, pharmaceutical compositions containing those compounds, and uses of the compounds and compositions as modulators of casein kinase 1 (e.g., CK1γ), casein kinase 2 (CK2), Pim 1, Pim2, Pim3, the TGFβ pathway, the Wnt pathway, the JAK/STAT pathway, and/or the mTOR pathway. Uses are also disclosed for the treatment or prevention of a range of therapeutic indications due at least in part to aberrant physiological activity of casein kinase 1 (e.g., CK1γ), casein kinase 2 (CK2), Pim 1, Pim2, Pim3, the TGFβ pathway, the Wnt pathway, the JAK/STAT pathway, and/or the mTOR pathway.
-
Synthesis of the Azepinoindole Framework via Oxidative Heck (Fujiwara-Moritani) Cyclization作者:Erik Van der Eycken、Pavel DonetsDOI:10.1055/s-0030-1260057日期:2011.7A catalytic oxidative Heck (Fujiwara-Moritani) cyclization has been evaluated for construction of the azepinoindole framework starting from readily available 3-indoleacetic acid amides. The supporting role of the amide group in the substrate has been demonstrated necessary for the success of the cyclization.
-
Combinations of substituted azetidinones and CB1 antagonists申请人:Veltri P. Enrico公开号:US20060069080A1公开(公告)日:2006-03-30The present invention provides compositions, therapeutic combinations and methods including: (a) at least one selective CB 1 antagonist; and (b) at least one substituted azetidinone or substituted β-lactam sterol absorption inhibitor which can be useful for treating vascular conditions, diabetes, obesity, metabolic syndrome and lowering plasma levels of sterols or 5α-stanols.
表征谱图
-
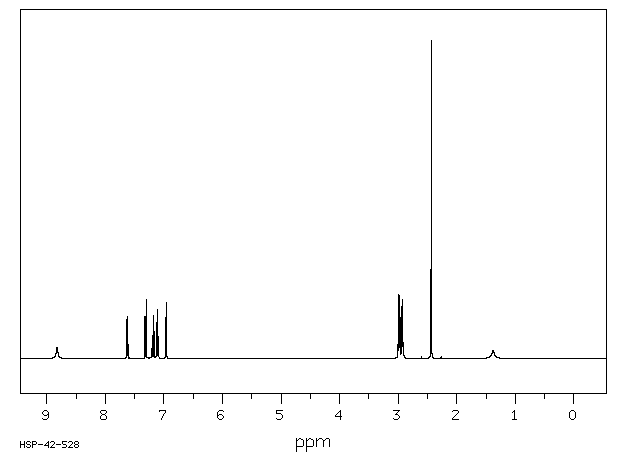
氢谱1HNMR
-
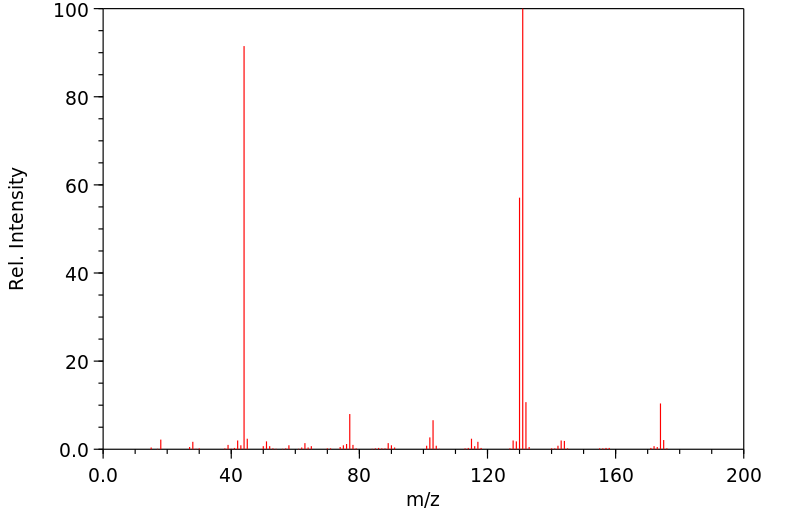
质谱MS
-
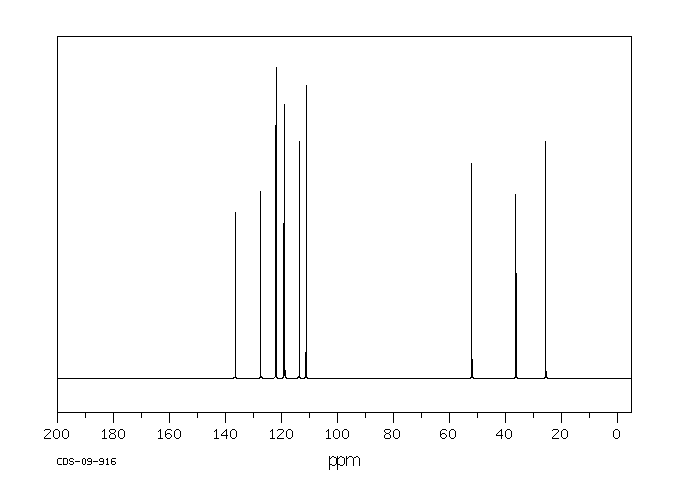
碳谱13CNMR
-
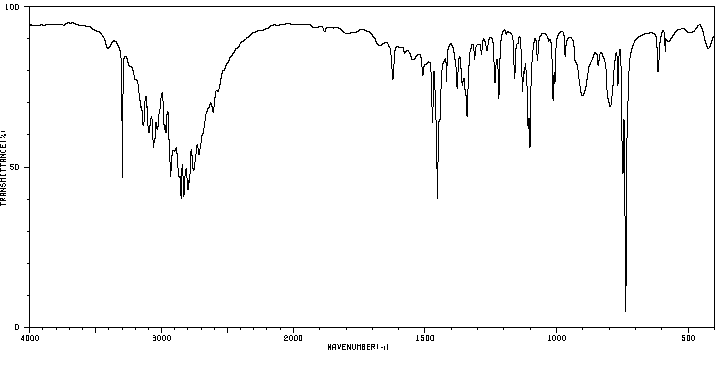
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Z)-3-[[[2,4-二甲基-3-(乙氧羰基)吡咯-5-基]亚甲基]吲哚-2--2-
(S)-(-)-5'-苄氧基苯基卡维地洛
(R)-(+)-5'-苄氧基卡维地洛
(R)-卡洛芬
(N-(Boc)-2-吲哚基)二甲基硅烷醇钠
(E)-2-氰基-3-(5-(2-辛基-7-(4-(对甲苯基)-1,2,3,3a,4,8b-六氢环戊[b]吲哚-7-基)-2H-苯并[d][1,2,3]三唑-4-基)噻吩-2-基)丙烯酸
(4aS,9bR)-6-溴-2,3,4,4a,5,9b-六氢-1H-吡啶并[4,3-B]吲哚
(3Z)-3-(1H-咪唑-5-基亚甲基)-5-甲氧基-1H-吲哚-2-酮
(3Z)-3-[[[4-(二甲基氨基)苯基]亚甲基]-1H-吲哚-2-酮
(3R)-(-)-3-(1-甲基吲哚-3-基)丁酸甲酯
(3-氯-4,5-二氢-1,2-恶唑-5-基)(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙酸
齐多美辛
鸭脚树叶碱
鸭脚木碱,鸡骨常山碱
鲜麦得新糖
高氯酸1,1’-二(十六烷基)-3,3,3’,3’-四甲基吲哚碳菁
马鲁司特
马鞭草(VERBENAOFFICINALIS)提取物
马来酸阿洛司琼
马来酸替加色罗
顺式-ent-他达拉非
顺式-1,3,4,4a,5,9b-六氢-2H-吡啶并[4,3-b]吲哚-2-甲酸乙酯
顺式-(+-)-3,4-二氢-8-氯-4'-甲基-4-(甲基氨基)-螺(苯并(cd)吲哚-5(1H),2'(5'H)-呋喃)-5'-酮
靛青二磺酸二钾盐
靛藍四磺酸
靛红联二甲酚
靛红磺酸钠
靛红磺酸
靛红乙烯硫代缩酮
靛红-7-甲酸甲酯
靛红-5-磺酸钠
靛红-5-磺酸
靛红-5-硫酸钠盐二水
靛红-5-甲酸甲酯
靛红
靛玉红衍生物E804
靛玉红3'-单肟5-磺酸
靛玉红-3'-单肟
靛玉红
靛噻
青色素3联己酸染料,钾盐
雷马曲班
雷莫司琼杂质13
雷莫司琼杂质12
雷莫司琼杂质
雷替尼卜定
雄甾-1,4-二烯-3,17-二酮
阿霉素的代谢产物盐酸盐
阿贝卡尔
阿西美辛杂质3