1,10-十一碳二烯 | 13688-67-0
中文名称
1,10-十一碳二烯
中文别名
1,10-十一二烯;1,10-十一烷二烯;1,1-十一碳二烯
英文名称
1,10-undecadiene
英文别名
Undecadien-(1,10);undeca-1,10-diene
CAS
13688-67-0
化学式
C11H20
mdl
MFCD00059203
分子量
152.28
InChiKey
VOSLXTGMYNYCPW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:187 °C
-
密度:0.77
-
保留指数:1067;1067;1088;1083;1085;1078
计算性质
-
辛醇/水分配系数(LogP):5.7
-
重原子数:11
-
可旋转键数:8
-
环数:0.0
-
sp3杂化的碳原子比例:0.636
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:3
-
危险品运输编号:3295
-
海关编码:2901299090
-
包装等级:III
-
危险类别:3
-
储存条件:存储条件:2~8℃,干燥
SDS
1,10-Undecadiene
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 1,10-Undecadiene
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 3
Flammable liquids
HEALTH HAZARDS
Category 1
Aspiration hazard
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements Flammable liquid and vapour
May be fatal if swallowed and enters airways
Precautionary statements:
Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
[Prevention]
Keep container tightly closed.
Use explosion-proof electrical/ventilating/lighting equipment. Take precautionary
measures against ignition by the static discharge and the spark.
Wear protective gloves/eye protection/face protection.
[Response] IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. Do NOT
induce vomiting.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
[Storage] Store in a well-ventilated place. Keep cool.
Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 1,10-Undecadiene
....
Percent:
CAS Number: 13688-67-0
Chemical Formula: C11H20
1,10-Undecadiene
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Keep containers cool by
spraying with water. Eliminate all ignition sources if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use extra personal protective equipment (self-contained breathing apparatus). Keep
Personal precautions,
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in dry sand or inert absorbent before recovering it into an
containment and cleaning airtight container. In case of large amount of spillage, contain a spill by bunding.
up: Adhered or collected material should be promptly disposed of, in accordance with
appropriate laws and regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from heat/sparks/open flame/hot
surfaces. -No smoking. Take measures to prevent the build up of electrostatic
charge. Use explosion-proof equipment. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Storage conditions:
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
1,10-Undecadiene
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Hand protection: Impervious gloves.
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Liquid
Physical state (20°C):
Form: Clear
Colour: Colorless - Slightly pale yellow
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 187°C
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 0.77
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Spark, Open flame, Static discharge
Conditions to avoid:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
1,10-Undecadiene
Section 12. ECOLOGICAL INFORMATION
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 3: Flammable liquid.
3295
UN-No:
Proper shipping name: Hydrocarbons, liquid, n.o.s.
Packing group: III
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 1,10-Undecadiene
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 3
Flammable liquids
HEALTH HAZARDS
Category 1
Aspiration hazard
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements Flammable liquid and vapour
May be fatal if swallowed and enters airways
Precautionary statements:
Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
[Prevention]
Keep container tightly closed.
Use explosion-proof electrical/ventilating/lighting equipment. Take precautionary
measures against ignition by the static discharge and the spark.
Wear protective gloves/eye protection/face protection.
[Response] IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. Do NOT
induce vomiting.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
[Storage] Store in a well-ventilated place. Keep cool.
Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 1,10-Undecadiene
....
Percent:
CAS Number: 13688-67-0
Chemical Formula: C11H20
1,10-Undecadiene
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Keep containers cool by
spraying with water. Eliminate all ignition sources if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use extra personal protective equipment (self-contained breathing apparatus). Keep
Personal precautions,
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in dry sand or inert absorbent before recovering it into an
containment and cleaning airtight container. In case of large amount of spillage, contain a spill by bunding.
up: Adhered or collected material should be promptly disposed of, in accordance with
appropriate laws and regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from heat/sparks/open flame/hot
surfaces. -No smoking. Take measures to prevent the build up of electrostatic
charge. Use explosion-proof equipment. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Storage conditions:
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
1,10-Undecadiene
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Hand protection: Impervious gloves.
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Liquid
Physical state (20°C):
Form: Clear
Colour: Colorless - Slightly pale yellow
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 187°C
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 0.77
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Spark, Open flame, Static discharge
Conditions to avoid:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
1,10-Undecadiene
Section 12. ECOLOGICAL INFORMATION
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 3: Flammable liquid.
3295
UN-No:
Proper shipping name: Hydrocarbons, liquid, n.o.s.
Packing group: III
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 10-十一烯-1-醇 10-Undecen-1-ol 112-43-6 C11H22O 170.295 (E)-十一碳-9-烯-1-醇 9-undecenol 112-46-9 C11H22O 170.295 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (Z)-cyclononene 933-21-1 C9H16 124.226 十一碳-10-烯-2-酮 undec-10-en-2-one 36219-73-5 C11H20O 168.279 —— (Z)-5,13-tetradecadien-1-ol 135346-30-4 C14H26O 210.36
反应信息
-
作为反应物:描述:参考文献:名称:Multiple Electron Tunneling Paths across Self-Assembled Monolayers of Alkanethiols with Attached Ruthenium(II/III) Redox Centers摘要:Alkanethiol monolayers with pendant redox centers are deposited on gold electrolytes by self-assembly. The monolayers are composed of both an electroactive thiol, HS(CH2)(n)C(O)NHCH(2)pyRu(NH3)(5)(2+/3+), with 10-15 methylene groups, and a diluent thiol, HS(CH2)mCoOH, also with 10-15 methylene groups. The monolayers are classified as ''matched'' (n = m), ''exposed'' (n = 15, m = 10-14), and ''buried'' (n = 10, m = 11-15) according to the relative position of the redox center. Cyclic voltammograms in aqueous Na2SO4 indicate that the monolayers are close-packed with the redox centers residing in the aqueous phase in all but the most buried cases. Measurements of electron transfer kinetics by several methods (cyclic voltammetry, ac impedance spectroscopy, chronoamperometry) yield an internally consistent set of kinetic parameters, the standard rate constant k degrees, and the reorganization energy lambda of the redox centers. The reorganization energies are in good agreement with the theoretically predicted value of 1.0 eV for the pyRu(NH3)(5) redox centers. Plots of ln(k degrees) vs m are linear in all three cases. The slopes of the linear regression fit provide tunneling parameters (beta, where k degrees approximate to e(-beta m)) of 0.97 +/- 0.03 (matched cases), 0.83 +/- 0.03 (exposed cases) and 0.16 +/- 0.02 (buried cases) per methylene. This pattern of beta's is interpreted in terms of electronic coupling between the redox center and the electrode via both the redox thiol and the proximate diluent thiols, with the coupling via the diluent thiols dominating in the exposed cases.DOI:10.1021/jp962831q
-
作为产物:描述:参考文献:名称:Mares,M.; Chvalovsky,V., Collection of Czechoslovak Chemical Communications, 1967, vol. 32, # 1, p. 382 - 397摘要:DOI:
文献信息
-
A FACILE TRANSFORMATION OF ESTER GROUPS TO VINYL GROUPS BY THE ELECTROREDUCTIVE METHOD作者:Tatsuya Shono、Yoshihiro Matsumura、Shigenori KashimuraDOI:10.1246/cl.1978.69日期:1978.1.5A facile method of the transformation of esters to vinyl compounds was developed by using the electroreductive 1,2-elimination as a key step. β-Hydroxysulfones were electrochemically reduced to vinyl compounds in good yields. Hydroxyolefins could be also prepared from the corresponding lactones.
-
ケイ素化合物及び化粧料申请人:信越化学工業株式会社公开号:JP2017019745A公开(公告)日:2017-01-26【課題】軽い感触で、伸びが良く、撥水性に優れ、化粧膜が均一であり、化粧品に使用されるシリコーン油、炭化水素油、及びエステル油等の各種油剤又は有機紫外線吸収剤との混合系でも経時安定性、化粧持続性に優れる化粧料を与えることができる新規なケイ素化合物を提供する。【解決手段】下記一般式(1)で示されるケイ素化合物。【化1】(式中、R1は炭素数1〜30のアルキル基、アリール基、アラルキル基及びフッ素置換アルキル基から選択される同種又は異種の有機基であり、R2は炭素数4〜20の置換又は非置換の直鎖状又は分岐状の2価炭化水素基であり、aは2〜3の整数である。)【選択図】なし
-
Absolute Configuration for 1,<i>n</i>-Glycols: A Nonempirical Approach to Long-Range Stereochemical Determination作者:Xiaoyong Li、Carmin E. Burrell、Richard J. Staples、Babak BorhanDOI:10.1021/ja2119767日期:2012.6.6n-glycols (n = 2-12, 16) bearing two chiral centers were rapidly determined via exciton-coupled circular dichroism (ECCD) using a tris(pentafluorophenyl)porphyrin (TPFP porphyrin) tweezer system in a nonempirical fashion devoid of chemical derivatization. A unique "side-on" approach of the porphyrin tweezer relative to the diol guest molecule is suggested as the mode of complexation.
-
Oxidation of terminal diols using an oxoammonium salt: a systematic study作者:Shelli A. Miller、James M. Bobbitt、Nicholas E. LeadbeaterDOI:10.1039/c7ob00039a日期:——A systematic study of the oxidation of a range of terminal diols is reported, employing the oxoammonium salt 4-acetamido-2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate (4-NHAc-TEMPO+ BF4−) as the oxidant. For substrates bearing a hydrocarbon chain of seven carbon atoms or more, the sole product is the dialdehyde. A series of post-oxidation reactions have been performed showing that the的范围内终端的二醇的氧化的系统研究报告,采用氧合氨盐4-乙酰氨基-2,2,6,6-四甲基哌-1-含氧铵四氟硼酸盐(4-NHAC-TEMPO + BF 4 -)作为氧化剂。对于带有七个或七个以上碳原子的烃链的底物,唯一的产物是二醛。已经进行了一系列的后氧化反应,表明由氧化步骤得到的产物混合物可以直接进行随后的转化。对于含有四个至六个碳原子的二醇,内酯产物是氧化后的主要产物。在1,2-乙二醇和1,3-丙二醇的情况下,当使用底物与氧化剂的化学计量比为1∶0.5时,形成相应的单醛,其与另外的二醇迅速反应以产生缩醛产物。考虑到它们使用其他方法制备的困难以及它们在进一步的反应化学中的潜在应用,这具有特别的合成价值。
-
Enzymatic Oxidative Tandem Decarboxylation of Dioic Acids to Terminal Dienes作者:Alexander Dennig、Sara Kurakin、Miriam Kuhn、Andela Dordic、Mélanie Hall、Kurt FaberDOI:10.1002/ejoc.201600358日期:2016.7The biocatalytic oxidative tandem decarboxylation of C7–C18 dicarboxylic acids to terminal C5–C16 dienes was catalyzed by the P450 monooxygenase OleT with conversions up to 29 % for 1,11-dodecadiene (0.49 g L–1). The sequential nature of the cascade was proven by the fact that decarboxylation of intermediate C6–C11 ω-alkenoic acids and heptanedioic acid exclusively gave nonconjugated 1,4-pentadiene;
表征谱图
-
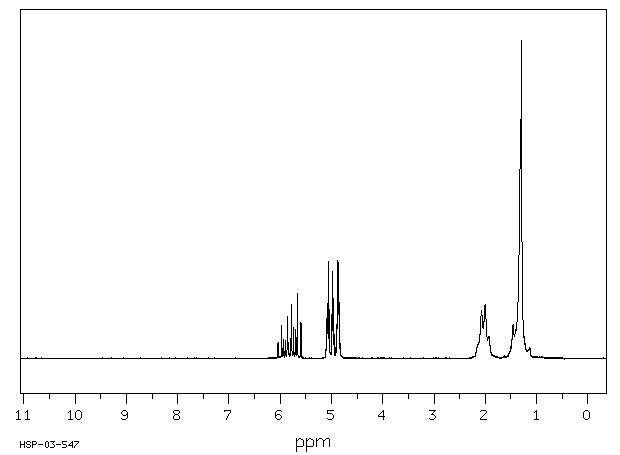
氢谱1HNMR
-
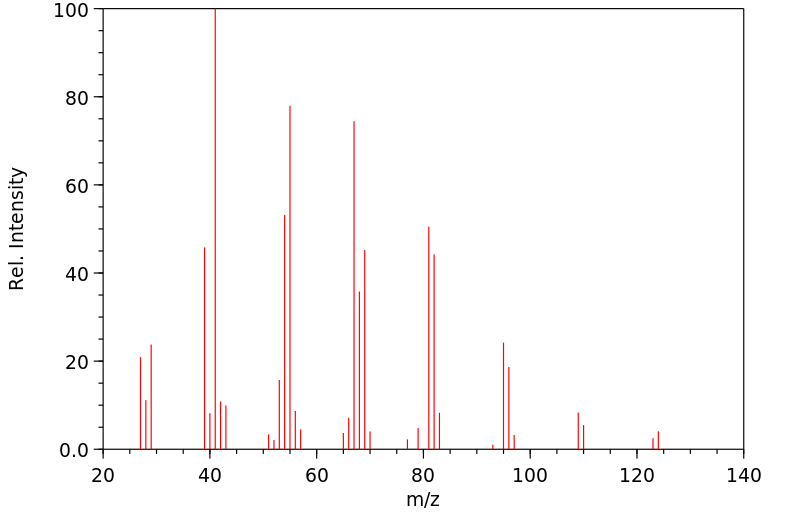
质谱MS
-
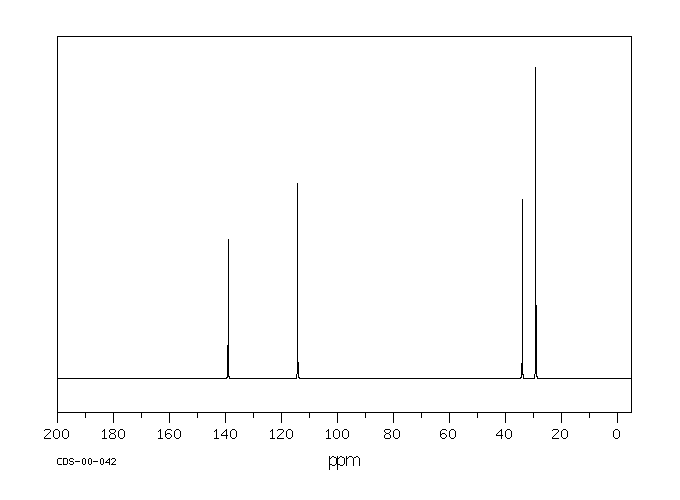
碳谱13CNMR
-
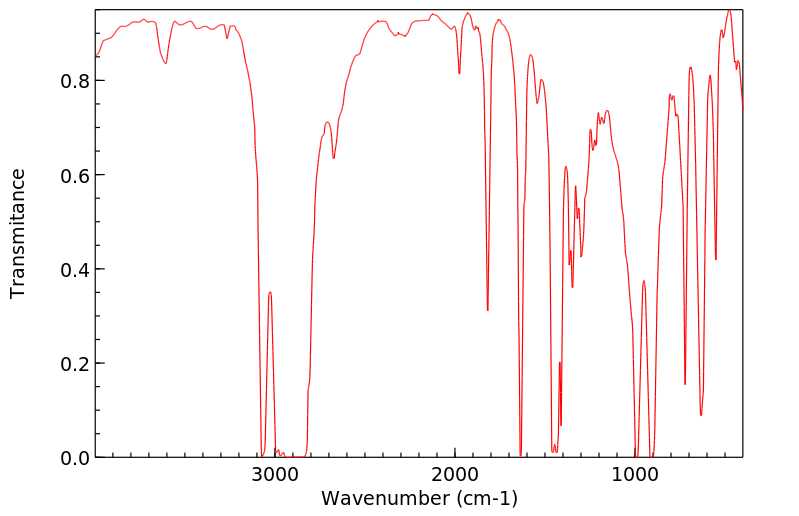
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-