顺-6-十三碳烯 | 6508-77-6
中文名称
顺-6-十三碳烯
中文别名
——
英文名称
(Z)-6-tridecene
英文别名
6-Tridecene;(Z)-tridec-6-ene
CAS
6508-77-6
化学式
C13H26
mdl
——
分子量
182.349
InChiKey
QHOMPCGOCNNMFK-QBFSEMIESA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:232.3±7.0 °C(Predicted)
-
密度:0.770±0.06 g/cm3(Predicted)
-
保留指数:1282;1271
计算性质
-
辛醇/水分配系数(LogP):6.3
-
重原子数:13
-
可旋转键数:9
-
环数:0.0
-
sp3杂化的碳原子比例:0.85
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Rove Beetle Aleochara pseudochrysorrhoa (Staphylinidae: Aleocharinae) 的 Tergal 腺分泌物:化学成分和生物学作用摘要:Aleochara pseudochrysorrhoa 有一个腺体复合体,称为 tergal 腺。通常,tergal 腺分泌物 (TGS) 已被描述为具有防御功能,但一些报告指出该复合体可能具有次要功能。例如,相关物种 A. curtula 的 TGS 已被证明在种内交流中具有重要作用。在这项工作中,我们描述了 A. pseudochrysorrhoa 男性和女性的 TGS 的化学成分。根据 GC/MS 和 GC-FT-IR 分析、保留指数和衍生产物鉴定了 11 种化合物。此外,还提供了关于 TGS 在交配行为中的生物学功能的简要研究,其中证明雌性 TGS 对雄性抓握反应的刺激依赖于浓度。DOI:10.1002/cbdv.202000483
-
作为产物:描述:参考文献:名称:Reactions of Organoboron Compounds with Phenyl(bromodichloromethyl)mercury. The Conversion of Cn-Terminal Olefins to C2n-1-Internal Olefins摘要:DOI:10.1021/ja00960a058
文献信息
-
Graphite oxide activated zeolite NaY: applications in alcohol dehydration作者:Alexander D. Todd、Christopher W. BielawskiDOI:10.1039/c2cy20474f日期:——zeolite NaY (Si/Al = 5.1) was used to dehydrate various alcohols to their respective olefinic products. Using conditions optimized for 4-heptanol (15 wt% GO–NaY (1 : 1 wt/wt), 150 °C, 30 min), a series of secondary and tertiary aliphatic alcohols were cleanly dehydrated in moderate to excellent conversions (27.5–97.2%). Several primary alcohols were also dehydrated, although higher catalyst loadings (200
-
Tunable stereoselective alkene synthesis by treatment of activated imines with nonstabilized phosphonium ylides作者:De-Jun Dong、Yuan Li、Jie-Qi Wang、Shi-Kai TianDOI:10.1039/c0cc04739b日期:——A broad range of readily accessible N-sulfonyl imines undergo olefination reaction with nonstabilized phosphonium ylides under mild conditions to afford an array of both Z- and E-isomers of 1,2-disubstituted alkenes, allylic alcohols, and allylic amines in good yields and with greater than 99 : 1 stereoselectivity.
-
Stabilization of long-chain intermediates in solution. Octyl radicals and cations作者:Aleksandar V. Teodorović、Dalibor M. Badjuk、Nenad Stevanović、Radoslav Z. PavlovićDOI:10.1016/j.molstruc.2013.02.020日期:2013.5The rearrangements of 1-octyl, 1-decyl and 1-tridecyl intermediates obtained from thermal lead(IV) acetate (LTA) decarboxylation of nonanoic, undecanoic and tetradecanoic acid were investigated experimentally through analysis and distribution of the products. The relationships between 1,5-, 1,6- and possibly existing 1,7-homolytic hydrogen transfer in 1-octyl-radical, as well as successive 1,2-hydride shift in corresponding cation have been computed via Monte-Carlo method. Taking into account that ratios of 1,5-/1,6-homolytic rearrangements in 1-octyl- and 1-tridecyl radical are approximately the same, the simulation shows very low involvement of 1,7-hydrogen rearrangement (1,5-/1,6-/1,7-hydrogen rearrangement = 85:31:1) in 1-octyl radical. (C) 2013 Elsevier B.V. All rights reserved.
-
Huhtasaari, Matti; Schaefer, Hans J.; Luftmann, Heinrich, Acta chemica Scandinavica. Series B: Organic chemistry and biochemistry, 1983, vol. 37, # 6, p. 537 - 548作者:Huhtasaari, Matti、Schaefer, Hans J.、Luftmann, HeinrichDOI:——日期:——
-
Liu, Hsing-Jang; Ho, Li-Kang, Canadian Journal of Chemistry, 1983, vol. 61, p. 632 - 634作者:Liu, Hsing-Jang、Ho, Li-KangDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
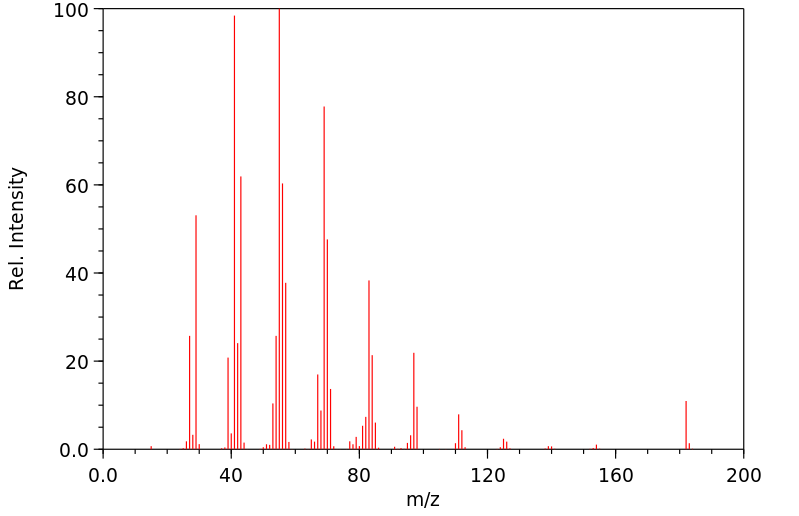
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-