顺式-1.1.1-三甲基-2-丁烯 | 762-63-0
中文名称
顺式-1.1.1-三甲基-2-丁烯
中文别名
——
英文名称
cis-4,4-dimethyl-2-pentene
英文别名
cis-4.4-Dimethyl-penten-(2);cis-4,4-Dimethyl-pent-2-en;cis-4,4-Dimethyl-2-penten;(Z)-4,4-dimethyl-2-pentene;4.4-Dimethyl-cis-penten-(2);(Z)-4,4-Dimethyl-2-penten;4,4-Dimethyl-cis-2-penten;(Z)-4,4-dimethylpent-2-ene
CAS
762-63-0
化学式
C7H14
mdl
——
分子量
98.1882
InChiKey
BIDIHFPLDRSAMB-WAYWQWQTSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-135.46°C
-
沸点:79.85°C
-
密度:0.6951
-
保留指数:644.9;642;642.8;644;646;648;645.1;646.9;643;643;639;643;645;638
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:7
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.71
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,3-二甲基-1-丁烯 t-butylethene 558-37-2 C6H12 84.1613 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 反式-4,4-二甲基-2-戊烯 (E)-4,4-dimethyl-2-pentene 690-08-4 C7H14 98.1882 —— 4,4-dimethyl-2-penten-1-ol 93667-58-4 C7H14O 114.188
反应信息
-
作为反应物:参考文献:名称:烯丙基型有机金属化合物的动力学和热力学控制的氧气触发构象平衡作用下烯基钾形成的可变立体选择性†摘要:(Z)-1-烷基化的丙烯在烯丙基部位的金属化反应比其(E)-异构体更快,无论在哪种构型下所得的有机金属在热力学上都更稳定。(Z)-4,4-二甲基-2-戊烯基钾给出了一个惊人的例证,其形成比其(E)-异构体快15倍,尽管后者在平衡条件下显然是有利的。烯基钾化合物在四氢呋喃溶液中的构型重组非常缓慢。然而,至少在一种情况下,微量的氧有效催化Z / E平衡。DOI:10.1002/hlca.19770600527
-
作为产物:描述:4c-tert-butyl-3r-methyl-2,2,2-triphenyl-2λ5-[1,2]oxaphosphetane 生成 顺式-1.1.1-三甲基-2-丁烯参考文献:名称:Low-temperature characterization of the intermediates in the Wittig reaction摘要:DOI:10.1021/ja00400a055
文献信息
-
Cis selectivity of salt-free Wittig reactions: a "Leeward Approach" of the aldehyde at the origin?作者:Manfred Schlosser、Bruno SchaubDOI:10.1021/ja00385a061日期:1982.10to afford, after 2-4 h, the corresponding olefins in 89-99% yield. In each case the trans isomer prevails, cis/trans ratios ranging from 33:67 to 4:96. The remarkable cis-stereoselectivity of Wittig reactions using triphenylphosphonium alkylides is now attributed to a very specific and almost rigid orientation of the Ph groups around the P atom, which sterically hinders approach to the aldehyde in
-
Novel<i>ortho</i>-Alkoxy-Substituted Phosphorus Ylides and Their Stereoselectivity in Witting Reactions作者:Suruliappa Jeganathan、Masamitsu Tsukarmoto、Manfred SchlosserDOI:10.1055/s-1990-26801日期:——The stereochemistry of the reactions between tris(2-methoxy-methoxyphenyl)phosphonioethanide (1f), -butanide (2f), and -phenyl-methanide (3f) and a variety of aldehydes was investigated. Ylides having a β-unbranched aliphatic sidechain, such as 2f, and saturated straight-chain aldehydes give olefins with unprecedented cis-selectivity (cis/trans â 200:1).
-
Binuclear Pd(I)–Pd(I) Catalysis Assisted by Iodide Ligands for Selective Hydroformylation of Alkenes and Alkynes作者:Yang Zhang、Sebastian Torker、Michel Sigrist、Nikola Bregović、Paweł DydioDOI:10.1021/jacs.0c09254日期:2020.10.21is driven by a novel activation strategy and features a unique Pd(I)-Pd(I) mechanism, involving an iodide-assisted binuclear step to release the product. This method enables β-selective hydroformylation of a large range of alkenes and alkynes, including sensitive starting materials. Its utility is demonstrated in the synthesis of antiobesity drug Rimonabant and anti-HIV agent PNU-32945. In a broader自 1938 年被发现以来,加氢甲酰化得到了彻底的研究并在工业中得到了广泛的应用(每年超过 107 公吨)。然而,迄今为止,使用成熟的 Rh 或助催化剂精确控制其区域选择性的能力已被证明是难以捉摸的,从而限制了许多具有合成价值的醛的获得。钯催化剂代表了一种有吸引力的替代品,但由于不希望的副加工,它们的使用仍然很少。在这里,我们报告了一种高选择性和异常活性的催化剂系统,该系统由一种新型活化策略驱动,并具有独特的 Pd(I)-Pd(I) 机制,涉及碘化物辅助双核步骤以释放产物。这种方法能够对大范围的烯烃和炔烃(包括敏感的起始材料)进行 β 选择性加氢甲酰化。它的效用在抗肥胖药物利莫那班和抗 HIV 药物 PNU-32945 的合成中得到了证明。在更广泛的背景下,新的机理理解使其他对化学工业具有重要意义的羰基化反应的发展成为可能。
-
Activation Parameters for the Epoxidation of Substitutedcis/trans Pairs of 1,2-Dialkylalkenes by Dimethyldioxirane作者:Brian S. Crow、W. Rucks Winkeljohn、Angela Navarro-Eisenstein、Elba Michelena-Baez、Paul J. Franklin、Pedro C. Vasquez、Al BaumstarkDOI:10.1002/ejoc.200600427日期:2006.10the corresponding cis isomers. The ΔH‡ terms mirrored trends observed in ΔG‡ because ΔS‡ terms for all ten of the compounds were roughly identical. The ΔΔG‡ values, a comparison of the trans to the cis isomer data, yielded positive values of 1.2 to 1.8 kcal/mol for the five sets of data and appeared to be dependent on relative steric interactions. The experimental activation parameter data, consistent报告了二甲基二环氧乙烷 (1) 与五个顺式/反式烯烃对反应的第一个活化参数数据。顺式-1,2-二烷基烯烃的环氧化(2顺式:R1 = Me,R2 = iPr;3顺式:R1 = Me,R2 = tBu;4顺式:R1 = R2 = Et;5顺式:R1 = Et,R2 = iPr;6顺式:R1 = Et,R2 = tBu)和反式-1,2-二烷基烯烃(2trans:R1 = Me,R2 = iPr;3trans:R1 = Me,R2 = tBu;4trans:R1 = R2 = Et;5trans:R1 = Et, R2 = iPr; 6trans: R1 = Et, R2 = tBu) 通过 1 产生相应的环氧化物,定量和立体定向,作为唯一可观察的产物。使用 Arrhenius 方法确定五对烯烃,2cis-6cis 和 2trans-6trans,1 环氧化的活化参数。在较低温度下观察到顺式与反式
-
Stereochemistry and regiochemistry of the addition of lodonium nitrate to alkenes作者:J. William Lown、Alummoottil V. JoshuaDOI:10.1039/p19730002680日期:——The stereochemistry of the products was confirmed by relating them chemically with known compounds. Addition to the less hindered (Z)-[β-2H]styrene is also stereospecific, eliminating the possibility of restricted rotation during addition. Ring closure by neighbouring sulphur in the addition of iodonium nitrate to 1-allyl-3,3-diethylthiourea affords a thiazole. The failure to obtain addition and phenyl
表征谱图
-
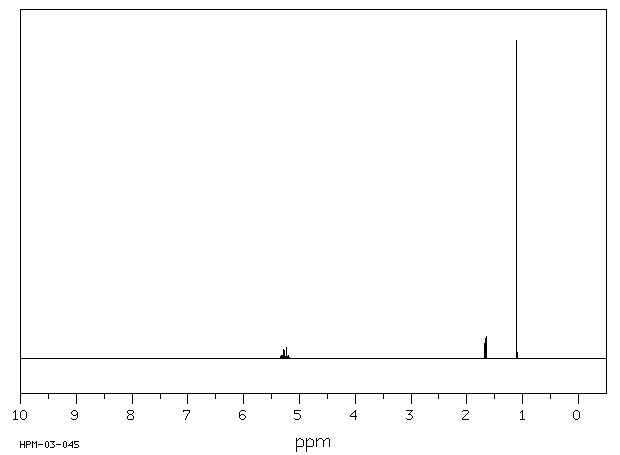
氢谱1HNMR
-
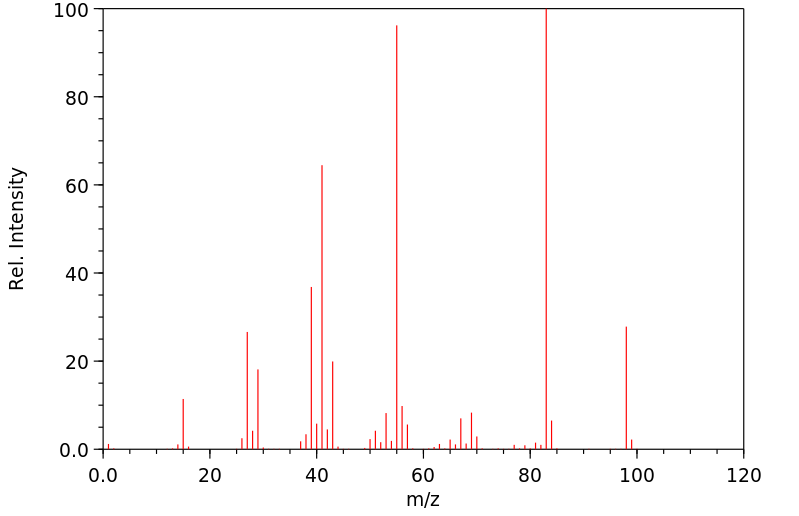
质谱MS
-
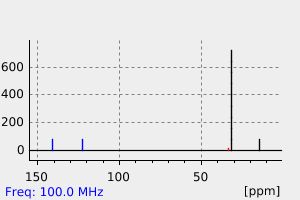
碳谱13CNMR
-
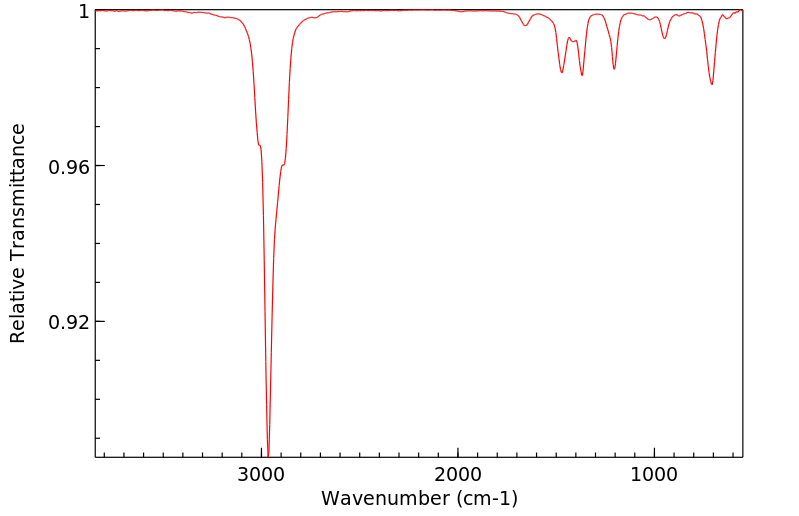
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-