顺式-3,4-二甲基-2-戊烯 | 4914-91-4
中文名称
顺式-3,4-二甲基-2-戊烯
中文别名
3,4-二甲-2-戊烯;顺-3,4-二甲基-2-戊烯;1-異丙-1,2-二甲基乙烯
英文名称
(Z)-3,4-dimethyl-2-pentene
英文别名
3,4-dimethyl-(Z)-2-pentene;cis-3,4-dimethyl-2-pentene;cis-3,4-dimethylpent-2-ene;3,4-dimethyl-Z-2-pentene;Z-3,4-dimethyl-2-pentene;3,4-dimethyl-2-pentene;(Z)-3,4-dimethylpent-2-ene
CAS
4914-91-4
化学式
C7H14
mdl
——
分子量
98.1882
InChiKey
PPBWEVVDSRKEIK-ALCCZGGFSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-113.39°C
-
沸点:87 °C
-
密度:0.71
-
闪点:-6 °C
-
保留指数:680.8;678.9;679.4;676;677;678;676.7;677.7;678;679;679;680;673;672;705.6;707;677;672.4;678;679;671
-
稳定性/保质期:
在常温常压下,该物质保持稳定。
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:7
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.71
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:3
-
危险品运输编号:UN 3295
-
储存条件:常温下应存于避光、阴凉干燥处,并密封保存。
SDS
cis-3,4-Dimethyl-2-pentene
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: cis-3,4-Dimethyl-2-pentene
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 2
Flammable liquids
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements Highly flammable liquid and vapour
Precautionary statements:
[Prevention] Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
Keep container tightly closed.
Use explosion-proof electrical/ventilating/lighting equipment. Take precautionary
measures against ignition by the static discharge and the spark.
Wear protective gloves/eye protection/face protection.
[Response] IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
[Storage] Store in a well-ventilated place. Keep cool.
Dispose of contents/container through a waste management company authorized by
[Disposal]
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: cis-3,4-Dimethyl-2-pentene
Percent: >98.0%(GC)
CAS Number: 4914-91-4
Chemical Formula: C7H14
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
cis-3,4-Dimethyl-2-pentene
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Keep containers cool by
spraying with water. Eliminate all ignition sources if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in dry sand or inert absorbent before recovering it into an
containment and cleaning airtight container. In case of large amount of spillage, contain a spill by bunding.
up: Adhered or collected material should be promptly disposed of, in accordance with
appropriate laws and regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from heat/sparks/open flame/hot
surfaces. -No smoking. Take measures to prevent the build up of electrostatic
charge. Use explosion-proof equipment. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
cis-3,4-Dimethyl-2-pentene
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Clear
Form:
Colour: Colorless - Almost colorless
No data available
Odour:
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 87°C
-6°C
Flash point:
Flammability or explosive
limits:
Lower: No data available
No data available
Upper:
Relative density: 0.71
Solubility(ies):
[Water] No data available
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Conditions to avoid: Spark, Open flame, Static discharge
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system but exert extra care in igniting as this material is
highly flammable. Observe all federal, state and local regulations when disposing of the substance
cis-3,4-Dimethyl-2-pentene
Section 14. TRANSPORT INFORMATION
Hazards Class: 3: Flammable liquid.
UN-No: 3295
Proper shipping name: Hydrocarbons, liquid, n.o.s.
Packing group: II
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: cis-3,4-Dimethyl-2-pentene
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 2
Flammable liquids
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements Highly flammable liquid and vapour
Precautionary statements:
[Prevention] Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
Keep container tightly closed.
Use explosion-proof electrical/ventilating/lighting equipment. Take precautionary
measures against ignition by the static discharge and the spark.
Wear protective gloves/eye protection/face protection.
[Response] IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
[Storage] Store in a well-ventilated place. Keep cool.
Dispose of contents/container through a waste management company authorized by
[Disposal]
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: cis-3,4-Dimethyl-2-pentene
Percent: >98.0%(GC)
CAS Number: 4914-91-4
Chemical Formula: C7H14
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
cis-3,4-Dimethyl-2-pentene
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Keep containers cool by
spraying with water. Eliminate all ignition sources if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in dry sand or inert absorbent before recovering it into an
containment and cleaning airtight container. In case of large amount of spillage, contain a spill by bunding.
up: Adhered or collected material should be promptly disposed of, in accordance with
appropriate laws and regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from heat/sparks/open flame/hot
surfaces. -No smoking. Take measures to prevent the build up of electrostatic
charge. Use explosion-proof equipment. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
cis-3,4-Dimethyl-2-pentene
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Clear
Form:
Colour: Colorless - Almost colorless
No data available
Odour:
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 87°C
-6°C
Flash point:
Flammability or explosive
limits:
Lower: No data available
No data available
Upper:
Relative density: 0.71
Solubility(ies):
[Water] No data available
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Conditions to avoid: Spark, Open flame, Static discharge
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system but exert extra care in igniting as this material is
highly flammable. Observe all federal, state and local regulations when disposing of the substance
cis-3,4-Dimethyl-2-pentene
Section 14. TRANSPORT INFORMATION
Hazards Class: 3: Flammable liquid.
UN-No: 3295
Proper shipping name: Hydrocarbons, liquid, n.o.s.
Packing group: II
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,4-二甲基-2-戊烯 (E)-3,4-dimethyl-2-pentene 4914-92-5 C7H14 98.1882 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3,4-二甲基-2-戊烯 (E)-3,4-dimethyl-2-pentene 4914-92-5 C7H14 98.1882 2-甲-3-亞甲基戊烷 2-ethyl-3-methyl-1-butene 7357-93-9 C7H14 98.1882
反应信息
-
作为反应物:描述:顺式-3,4-二甲基-2-戊烯 在 C30H42IrNOP(1+)*C32H12BF24(1-) 、 氢气 作用下, 以 二氯甲烷 为溶剂, 20.0 ℃ 、5.0 MPa 条件下, 反应 4.0h, 生成 (R)-2,3-dimethyl-pentane参考文献:名称:铱催化的未官能化三烷基取代烯烃的不对称加氢反应摘要:具有双环吡啶基N,P配体的手性铱配合物已成为未官能化三烷基取代烯烃对映选择性加氢的有效催化剂。通过改变溶剂,压力和温度来优化反应条件,导致对映体过量高达99%。将(E)-2-环己基-2-丁烯和(E)-和(Z)-3,4-二甲基-2-戊烯这三种纯烯烃分别转化为相应的手性烷烃,分别为97%,94%和93 % ee, 分别。醋酸α-和γ-生育三烯基酯的三个C bondsC键的加氢导致醋酸α-和γ-生育酚酯具有非常高的非对映选择性。相同的催化剂已成功用于加氢取代带有γ位羧酸酯或酮基的三取代烯烃。该反应被用作关门菌西双螺旋菌的信息素的高度对映选择性合成中的关键步骤。结构类似的烯丙基醇的氢化也给出了高对映选择性。DOI:10.1002/asia.201000595
-
作为产物:描述:参考文献:名称:Crombie,L. et al., Journal of the Chemical Society. Perkin transactions I, 1975, p. 1081 - 1090摘要:DOI:
文献信息
-
THE ENE REACTION OF TRISUBSTITUTED ALKENES WITH ELECTRON-DEFICIENT NITRILES IN THE PRESENCE OF BORON TRICHLORIDE作者:Hiroshi Hamana、Tsutomu SugasawaDOI:10.1246/cl.1985.575日期:1985.5.5The ene reaction of trisubstituted alkenes with electron-deficient nitriles proceeds smoothly in the presence of boron trichloride to afford β,γ-unsaturated ketones in high yields. Investigation of the stereochemistry of the ene products suggests that this ene reaction is a concerted one. Similar reactions of cyclic trisubstituted alkenes are also described.
-
Rearrangement of cyclopropyl, substituted vinyl and alkyl groups to divalent carbon.作者:A.R. Kraska、L.I. Cherney、H. ShechterDOI:10.1016/s0040-4039(00)87289-5日期:1982.1The relative carbenic migratory abilities of cyclopropyl, β,β-dimenthylvinyl, methyl, ethyl and isopropyl group have been determined.已经确定了环丙基,β,β-二薄荷基乙烯基,甲基,乙基和异丙基的相对羧迁移能力。
-
One-step hydroprocessing of fatty acids into renewable aromatic hydrocarbons over Ni/HZSM-5: insights into the major reaction pathways作者:Shiyou Xing、Pengmei Lv、Jiayan Wang、Junying Fu、Pei Fan、Lingmei Yang、Gaixiu Yang、Zhenhong Yuan、Yong ChenDOI:10.1039/c6cp06327f日期:——Considerable amounts of AHCs, up to 64.61 wt%, were produced through the one-step hydroprocessing of fatty acids over Ni/HZSM-5 catalysts. Hydrogenation, hydrocracking, and aromatization constituted the principal AHC formation processes. At a lower temperature, fatty acids were first hydrosaturated and then hydrodeoxygenated at metal sites to form long-chain hydrocarbons. Alternatively, the unsaturated fatty对于生物航空燃料中的高热量和稳定性,至关重要的是一定含量的芳烃(AHC,8–25 wt%)。从废油或不可食用的油中获得的脂肪酸是用于AHC生产的可再生经济原料。通过在Ni / HZSM-5催化剂上对脂肪酸进行一步加氢处理,可生产出数量高达64.61 wt%的AHC。加氢,加氢裂化和芳构化是AHC的主要形成过程。在较低的温度下,首先将脂肪酸加氢饱和,然后在金属位点加氢脱氧以形成长链烃。或者,不饱和脂肪酸可在酸位点直接脱氧而不先饱和。长链烃裂化为乙烷,丙烷和C 6 -C等气体催化剂的布朗斯台德酸位上有8个烯烃;这些在催化剂的路易斯酸位上进行了狄尔斯-阿尔德反应,形成了AHC。C 6 -C 8烯烃被确定为AHC形成的关键中间体。随着催化剂中Ni含量的增加,布朗斯台德酸位点密度由于被金属纳米颗粒覆盖而降低。负载10%(重量)的Ni可获得良好的性能,其中Ni纳米颗粒表现出多面体形态,暴露出更多的芳构化活性位。
-
High Yield of Liquid Range Olefins Obtained by Converting <i>i</i>-Propanol over Zeolite H-ZSM-5作者:Uffe V. Mentzel、Saravanamurugan Shunmugavel、Sarah L. Hruby、Claus H. Christensen、Martin S. HolmDOI:10.1021/ja907692t日期:2009.11.25Methanol, ethanol, and i-propanol were converted under methanol-to-gasoline (MTH)-like conditions (400 degrees C, 1-20 bar) over zeolite H-ZSM-5. For methanol and ethanol, the catalyst lifetimes and conversion capacities are comparable, but when i-propanol is used as the reactant, the catalyst lifetime is increased dramatically. In fact, the total conversion capacity (calculated as the total amount
-
Steric Effects in Photoinduced Electron Transfer Reaction of Halogenated 1,4-Benzoquinones with Donor Olefins作者:Ken Kokubo、Takayoshi Masaki、Takumi OshimaDOI:10.1021/ol006080t日期:2000.6.1The photoinduced electron transfer reaction of halogenated 1,4-benzoquinones with 2,3-dimethyl-2-butene or 3,4-dimethyl-2-pentene gave the primary, secondary, and tertiary monoallyl ethers of hydroquinones, with the product distributions highly dependent on the steric nature of quinones and olefins.
表征谱图
-
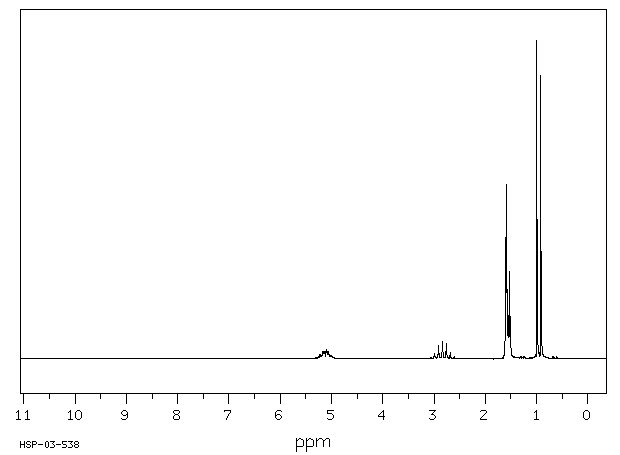
氢谱1HNMR
-
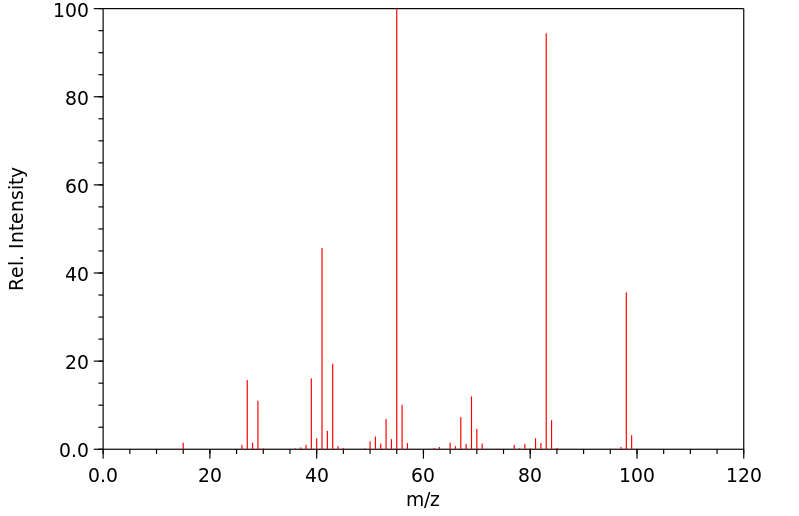
质谱MS
-
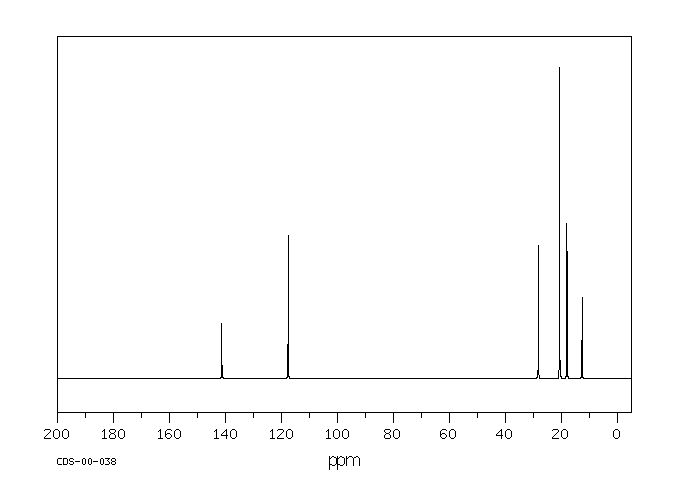
碳谱13CNMR
-
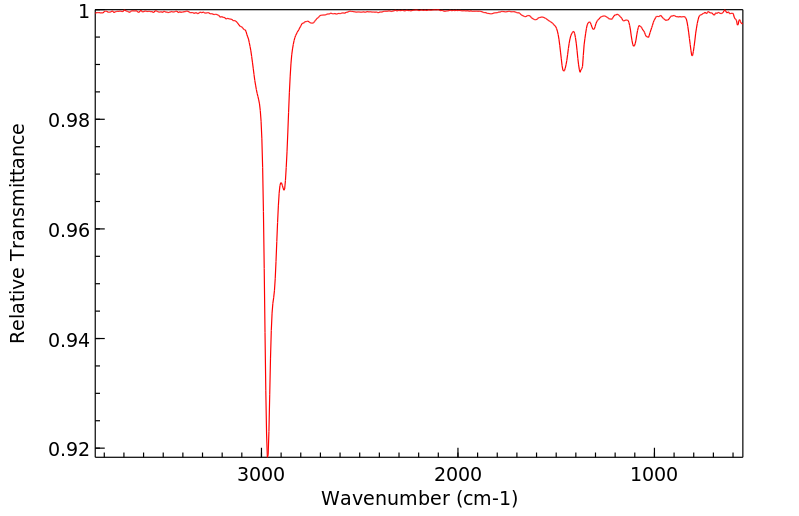
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-