香叶醇 | 16736-42-8
中文名称
香叶醇
中文别名
2,7-二甲基-2,6-辛二烯
英文名称
2,7-dimethyl-octa-2,6-diene
英文别名
2,7-dimethyl-2,6-octadiene;2,7-dimethylocta-2,6-diene
CAS
16736-42-8
化学式
C10H18
mdl
——
分子量
138.253
InChiKey
PSOPUECGKNQIPH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-74.4°C
-
沸点:229-230 °C(lit.)
-
密度:0.879 g/mL at 20 °C(lit.)
-
蒸气密度:5.31 (vs air)
-
闪点:216 °F
-
稳定性/保质期:
存在于主流烟气中。
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:10
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
WGK Germany:1
-
RTECS号:RG5830000
-
海关编码:2905221000
-
安全说明:S26,S36
SDS
上下游信息
反应信息
-
作为反应物:描述:香叶醇 在 chloroborane methyl sulfide complex 、 sodium hydroxide 、 双氧水 作用下, 以 四氢呋喃 、 水 为溶剂, 反应 1.5h, 生成 2,7-二甲基-3,6-辛二醇参考文献:名称:(±)-trans-2,5-二异丙基硼烷的合成摘要:研究了 2,7-二甲基-2,6-辛二烯 (6) 的环状硼氢化反应。发现反应的立体化学结果取决于溶剂、温度、时间和硼烷试剂的性质。在顺式-2,5-二异丙基硼烷(15)与1-(2-羟乙基)-吡咯烷选择性络合后分离纯外消旋反式-2,5-二异丙基硼烷(14)。DOI:10.3390/60300244
-
作为产物:参考文献:名称:The Substrate Specificity ofβ,β-Carotene 15,15′-Monooxygenase摘要:The synthesis of several substrate analogues of the enzyme beta,beta -carotene 15,15'-monooxygenase is reported. The substrate specificity of enriched enzyme fractions isolated from chicken intestinal mucosa was investigated. Regarding substrate binding/cleavage, these experiments demonstrate that i) any deviation from the 'rod-like' beta,beta -carotene structure is not tolerated, ii) one 'natural', unsubstituted beta -ionone ring is required, iii) the position and presence of the Me groups attached to the polyene chain is significant. These results suggest a hydrophobic barrel-like substrate binding site in which the protein's amino acid residues through interaction with the Me groups, direct the central C=C bond in binding distance to the active site's metal-oxo center, supporting the unique regiospecificity of cleavage to retinal (provitamin A).DOI:10.1002/1522-2675(20010815)84:8<2301::aid-hlca2301>3.0.co;2-u
文献信息
-
Catalyst-Free Suzuki-Type Coupling of Allylic Bromides with Arylboronic Acids作者:Alberto Scrivanti、Valentina Beghetto、Matteo Bertoldini、Ugo MatteoliDOI:10.1002/ejoc.201101527日期:2012.1The coupling of arylboronic acids with electron-rich allylic bromides is accomplished in the absence of any transition-metal catalyst through conventional heating. The reaction is completely regioselective, affording only the α-coupled product, and can be carried out under mild aerobic conditions in an organic solvent; the presence of a base is required.
-
CARBON–CARBON BOND FORMATION WITH METALLIC MANGANESE
-
New electrochemical synthesis of ketones from organic halides and carbon monoxide作者:Maïténa Oçafrain、Marguerite Devaud、Michel Troupel、Jacques PérichonDOI:10.1039/c39950002331日期:——The dissolution of a stainless steel anode provides catalytic nickel species which enable the efficient synthesis of ketones by electrolysis of organic halides in DMF in the presence of bipyridine and carbon monoxide.
-
Reductive Dimerization of Allyl and Benzyl Halides in Pb/<i>n</i>-Bu<sub>4</sub>NBr–DMF and PbBr<sub>2</sub>/Al–DMF Systems作者:Hideo Tanaka、Shiro Yamashita、Sigeru ToriiDOI:10.1246/bcsj.60.1951日期:1987.5Reductive dimerization of allyl and benzyl halides has been performed by treatment with Pb/n-Bu4NBr and catalytic PbBr2/Al in N,N-dimethylformamide.使用Pb/n-Bu4NBr处理以及以PbBr2/Al为催化剂,在N,N-二甲基甲酰胺中实现了烯丙基卤代物和苄基卤代物的还原二聚反应。
-
Highly Selective Carbon-Carbon Bond Forming Reactions Mediated by Chromium(II) Reagents作者:Tamejiro Hiyama、Yoshitaka Okude、Keizo Kimura、Hitosi NozakiDOI:10.1246/bcsj.55.561日期:1982.2to produce unisolable allylchromium species which add efficiently to aldehydes or ketones with high degree of stereo- and chemoselectivity. Particularly, high threo selectivity is observed in the reaction of aldehydes and 1-bromo-2-butene and is ascribed to a chair-like six-membered transition state. Simple reduction of allylic and benzylic halides produces biallyls and bibenzyls, while gem-dibromocyclopropanes
表征谱图
-
氢谱1HNMR
-
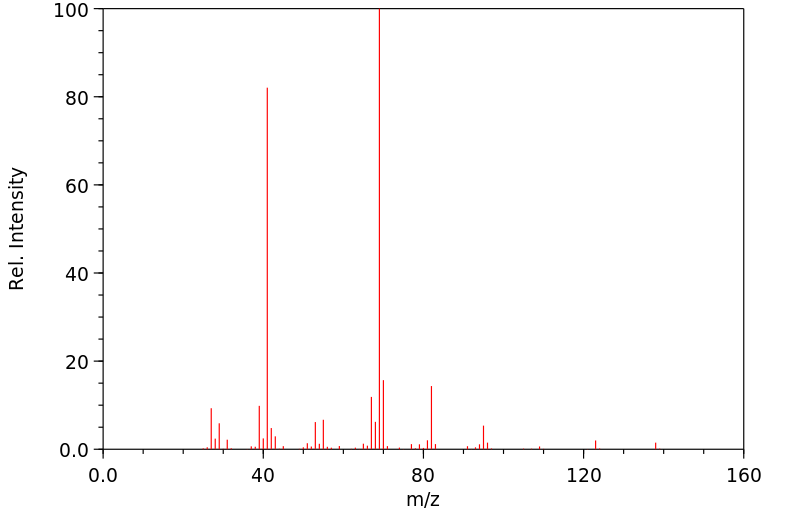
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-