邻甲酚 | 95-48-7
物质功能分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:29-31 °C (lit.)
-
沸点:191 °C (lit.)
-
密度:1.048 g/mL at 25 °C
-
蒸气密度:3.72 (vs air)
-
闪点:178 °F
-
溶解度:20g/l
-
暴露限值:NIOSH REL: TWA 2.3 ppm (10 mg/m3), IDLH 250 ppm; OSHA PEL: TWA 5 ppm (22 mg/m3); ACGIH TLV: TWA for all isomers 5 ppm (adopted).
-
介电常数:5.8(-5℃)
-
LogP:1.95 at 20℃
-
物理描述:O-cresol appears as colorless or yellow to brown-yellow or pinkish colored liquid with a phenol-like odor. Toxic by ingestion and/or skin absorption. May have a flash point between 100 and 199°F. Causes burns to skin, eyes and mucous membranes. Insoluble in water.
-
颜色/状态:Crystals in liquid becoming dark with age and exposure to light and air
-
气味:Phenolic odor
-
蒸汽密度:3.72 (EPA, 1998) (Relative to Air)
-
蒸汽压力:0.18 mm Hg at 25 °C
-
亨利常数:Henry's Law constant = 1.2X10-6 atm-cu m/mol at 25 °C
-
大气OH速率常数:4.20e-11 cm3/molecule*sec
-
稳定性/保质期:
-
遇明火、高热或氧化剂能引起燃烧。邻甲酚具有弱酸性,与氢氧化钠作用生成可溶性的钠盐,但不与碳酸钠反应。它还能与烷基化剂如硫酸二甲酯反应生成酚醚,并与醛类反应形成合成树脂。在温和条件下,邻甲酚可以进行硝化、卤化、烷基化和磺化等多种化学反应。此外,邻甲酚容易氧化,在光照下会变深色并生成醌类等复杂化合物。
-
邻甲酚有毒,主要影响中枢神经系统,严重时可致死。吸入或皮肤接触其蒸气或烟雾可能导致慢性肾炎和神经障碍。工作场所空气中允许的最大浓度为5×10^-6。大鼠口服的半数致死量为1350毫克/千克。操作过程中需确保设备密封,并配备防护用具。
-
邻甲酚在常规条件下较为稳定。
-
应避免与强氧化剂和碱类接触。
-
应避免光照。
-
邻甲酚不会发生聚合反应。
-
-
自燃温度:1110 °F (599 °C)
-
粘度:3.035 cP at 50 °C; 1.562 cP at 75 °C; 0.961 cP at 100 °C
-
燃烧热:-3696 kJ/mol at 25 °C
-
汽化热:45.19 kJ/mol at 191.04 °C
-
表面张力:36.90 mN/m at 25 °C; 34.38 mN/m at 50 °C
-
电离电位:8.93 eV
-
气味阈值:Odor Threshold Low: 0.26 [mmHg]; Odor Threshold High: 0.65 [mmHg]; Odor threshold low (detection) and high (recognition) from CHRIS
-
折光率:Index of refraction: 1.553 at 20 °C/D
-
解离常数:pKa = 10.287
-
保留指数:1031.1 ;1029.7 ;1032 ;1042 ;1044 ;1029 ;1039 ;1027 ;1032 ;1032 ;1032 ;1014.3 ;1017.6 ;1007 ;1008 ;1029 ;1030 ;1040 ;1048 ;1035 ;1078 ;1034 ;1034 ;1037 ;1030 ;1033 ;1023.6 ;1030 ;1027 ;1038 ;1039.1 ;1030 ;1036 ;1035.2 ;1035 ;1024 ;1026.3 ;1024 ;1035 ;1058 ;172.86 ;173.57
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
ADMET
安全信息
-
职业暴露等级:B
-
职业暴露限值:TWA: 2.3 ppm (10 mg/m3)
-
TSCA:Yes
-
危险等级:6.1
-
立即威胁生命和健康浓度:250 ppm
-
危险品标志:T
-
安全说明:S36/37/39,S45
-
危险类别码:R34,R24/25
-
WGK Germany:1,2
-
海关编码:29071200
-
危险品运输编号:UN 2076/3455
-
危险类别:6.1
-
RTECS号:GO6300000
-
包装等级:II
-
储存条件:储存注意事项:应将物品储存于阴凉、通风的库房中,并远离火种和热源。包装需密封,避免与空气接触。务必与氧化剂、碱类及食用化学品分开存放,严禁混储。配备相应种类和数量的消防器材是必要的。储存区域还应备有合适的材料以处理可能发生的泄漏。
SDS
| 国标编号: | 61073 |
| CAS: | 95-48-7 |
| 中文名称: | 2-甲(苯)酚 |
| 英文名称: | 2-methylphenol;o-Cresol |
| 别 名: | 邻甲(苯)酚 |
| 分子式: | C 7 H 8 O;HOC 6 H 4 CH 3 |
| 分子量: | 108.13 |
| 熔 点: | 30.8℃ 沸点:190.8℃ |
| 密 度: | 相对密度(水=1)1.05; |
| 蒸汽压: | 81℃ |
| 溶解性: | 微溶于水,溶于乙醇、乙醚、氯仿等 |
| 稳定性: | 稳定 |
| 外观与性状: | 白色结晶,有芳香气味 |
| 危险标记: | 14(有毒品) |
| 用 途: | 用作分析试剂并用于有机合成 |
2.对环境的影响:
一、健康危害
侵入途径:吸入、食入、经皮吸收。
健康危害:本品对皮肤、粘膜有强烈刺激和腐蚀作用。引起多脏器损害。
急性中毒:引起肌肉无力、胃肠道症状、中枢神经抑制、虚脱、体温下降和昏迷,并可引起肺水肿和肝、肾、胰等脏器损害,最终发生呼吸衰竭。
慢性影响:可引起消化道功能障碍,肝、肾损害和皮疹。
二、毒理学资料及环境行为
毒性:属低毒类。
急性毒性:LD 50 121mg/kg(大鼠经口);890mg/kg(兔经皮)
刺激性:家兔经皮:12500μg(24小时),轻度刺激。家兔经眼:100mg,轻度刺激。
致癌性:小鼠经皮最低中毒剂量(TDL 0 ):4800mg/kg(12周,间歇),致肿瘤阳性。
对生物降解的影响:水中浓度11~16mg/L时,活性污泥对氨氮的硝化作用降低75%,浓度50mg/L时,荧光假单孢菌对葡萄糖的降解受到抑制,浓度600mg/L时,大肠杆菌对葡萄糖的降解受到抑制。
危险特性:遇明火、高热或与氧化剂接触,有引起燃烧爆炸的危险。具有腐蚀性。
燃烧(分解)产物:一氧化碳、二氧化碳。
3.现场应急监测方法:
4.实验室监测方法:
| 监测方法 | 来源 | 类别 |
| 气相色谱法 | 《空气和废气监测分析方法》国家环保局编 | 空气和废气 |
| 气相色谱法;色谱/质谱法 | 《固体废弃物试验分析评价手册》中国环境监测总站等译 | 固体废弃物 |
| 高效液相色谱法 | 《空气中有害物的测定方法》(第二版),杭士平主编 | 空气 |
| 氯亚胺二溴苯醌比色法 | 《化工企业空气中有害物质测定方法》,化学工业出版社 | 化工企业空气 |
5.环境标准:
| 中国(TJ36-79) | 车间空气中有害物质的最高容许浓度 | 5mg/m 3 |
| 前苏联(1978) | 环境空气中基本安全浓度 | 28μg/m 3 |
| 前苏联(1975) | 水体中有害物质最高允许浓度 | 0.05mg/L |
| 前苏联(1978) | 渔业水中最高允许浓度 | 3μg/L |
| 前苏联(1975) | 污水排放标准 | 0.1mg/L |
| 嗅觉阈浓度 | 0.00068ppm |
6.应急处理处置方法:
一、泄漏应急处理
隔离泄漏污染区,限制出入。切断火源。建议应急处理人员戴自给式呼吸器,穿防毒服。不要直接接触泄漏物。小量泄漏:避免扬尘,用洁净的铲子收集于干燥、洁净、有盖的容器中。大量泄漏:收集回收或运至废物处理场所处置。
废弃物处置方法:用焚烧法。
二、防护措施
呼吸系统防护:空气中粉尘浓度超标时,应该佩戴头罩型电动送风过滤式防尘呼吸器;可能接触其蒸气时,应该佩戴自吸过滤式防毒面具(全面罩)。
眼睛防护:呼吸系统防护中已作防护。
身体防护:穿胶布防毒衣。
手防护:戴橡胶手套。
其它:工作现场禁止吸烟、进食和饮水。工作毕,彻底清洗。单独存放被毒物污染的衣服,洗后备用。注意个人清洁卫生。
三、急救措施
皮肤接触:立即脱去被污染的衣着,用甘油、聚乙烯乙二醇或聚乙烯乙二醇和酒精混合液(7 : 3)抹洗,然后用水彻底清洗。或用大量流动清水冲洗,至少15分钟。就医。
眼睛接触:立即提起眼睑,用大量流动清水或生理盐水彻底冲洗至少15分钟。就医。
吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。如呼吸停止,立即进行人工呼吸。就医。
食入:立即给饮植物油15-30mL。催吐。就医。
灭火方法:消防人员须佩戴防毒面具、穿全身消防服。灭火剂:雾状水、泡沫、干粉、二氧化碳、砂土。
制备方法与用途
概述 邻甲酚又名邻甲苯酚。它是一种无色液体或结晶体,能与醇、醚及氯仿混溶,并可溶解于氢氧化钠溶液中,但在水中微溶。其具有类似苯酚的气味,在暴露于空气和日光下会变暗且有毒。这种物质主要作用于中枢神经,严重时可能导致生命危险。
应用 邻甲酚主要用于生产除草剂二甲四氮、癸二酸稀释剂、消毒剂及医药中间体等,同时也可用于树脂制造、增塑剂合成、香料与染料的制作以及检验硝酸盐和砷酸分析所需的试剂。此外,它也可作为食用香精的一部分。
食品添加剂最大允许使用量 根据GB 2760-1996规定,邻甲酚被列为允许使用的食用香料,并且其在食品中的最大使用量及残留量需遵循该标准。
化学性质 无色结晶体,散发苯酚气味。它能溶于约40倍的水中(3%至5.3%,具体取决于温度),并几乎全部溶解于苛性碱液以及各种常用有机溶剂中。
用途 邻甲酚是合成香豆素的重要中间体,还可用作消毒剂和防腐剂,在癸二酸生产过程中用作稀释剂。此外,它亦用于有机合成及分析试剂的制备等。
生产方法 从煤焦化所得的酚油切取含有6%邻甲酚、49.4%间甲酚与32.1%对甲酚以及少量二甲酚和苯酚的馏分。通过蒸馏分离,可在不低于40块理论塔板的精馏柱内获得纯度大于97%的邻甲酚。
合成方法包括:
生产方法 由粗酚预脱水初馏后进行减压精馏以获取目标产品。该过程采用固定床列管式反应器,在特定条件下制备邻甲酚,再通过分馏分离、蒸馏提纯等步骤获得高纯度产物。
类别与危险性 邻甲酚属于腐蚀物品且为高毒物质。急性毒性测试表明,其LD50值分别为大鼠121毫克/千克和小鼠344毫克/千克。皮肤接触或眼睛刺激实验显示具有强烈反应;遇空气高温可燃,并能产生刺激烟雾。
储运与灭火 应存储于通风良好、干燥且低温环境并远离氧化剂,采用泡沫或雾状水进行扑灭火灾。
职业暴露标准 时间加权平均容许浓度(TWA)为22毫克/立方米;短期接触极限( STEL )为44毫克/公斤。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,6-二甲基苯酚 2,6-xylenol 576-26-1 C8H10O 122.167 2,4-二甲基苯酚 2,4-Xylenol 105-67-9 C8H10O 122.167 对甲酚 p-cresol 106-44-5 C7H8O 108.14 2-甲基苯甲醚 2-methylmethoxybenzene 578-58-5 C8H10O 122.167 水杨醛 salicylaldehyde 90-02-8 C7H6O2 122.123 间甲酚 3-methyl-phenol 108-39-4 C7H8O 108.14 水杨醇 salicylic alcohol 90-01-7 C7H8O2 124.139 邻羟基苯甲腈 2-Cyanophenol 611-20-1 C7H5NO 119.123 3-甲基苯邻二酚 3-methylbenzene-1,2-diol 488-17-5 C7H8O2 124.139 4-氯-2-甲基苯酚 2-methyl-4-chlorophenol 1570-64-5 C7H7ClO 142.585 4-碘-2-甲基苯酚 4-iodo-2-methyl-phenol 60577-30-2 C7H7IO 234.036 4-氟-2-甲基苯酚 4-fluoro-2-methylphenol 452-72-2 C7H7FO 126.13 4-溴-2-甲基苯酚 4-Bromo-2-methylphenol 2362-12-1 C7H7BrO 187.036 2-甲基苯基甲酸酯 2-methylphenyl formate 1864-95-5 C8H8O2 136.15 2-(甲氧基甲基)苯酚 2-(methoxymethyl)phenol 5635-98-3 C8H10O2 138.166 邻乙氧基甲苯 2-ethoxytoluene 614-71-1 C9H12O 136.194 水杨醛腙 2-(hydrazonomethyl)phenol 3291-00-7 C7H8N2O 136.153 异丙苯酚 2-(1-methylethyl)phenol 88-69-7 C9H12O 136.194 2-氯-6-甲酚 2-Chloro-6-methylphenol 87-64-9 C7H7ClO 142.585 对羟基苯甲醛 4-hydroxy-benzaldehyde 123-08-0 C7H6O2 122.123 间羟基苯甲醛 meta-hydroxybenzaldehyde 100-83-4 C7H6O2 122.123 1-甲基-2-(苯氧基)苯 1-methyl-2-phenoxybenzene 3991-61-5 C13H12O 184.238 苯甲基苯酚 o-Benzylphenol 28994-41-4 C13H12O 184.238 - 1
- 2
- 3
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,6-二羟基甲苯 2-Methylresorcinol 608-25-3 C7H8O2 124.139 2,6-二甲基苯酚 2,6-xylenol 576-26-1 C8H10O 122.167 甲基氢醌 2-methylbenzene-1,4-diol 95-71-6 C7H8O2 124.139 2,5-二甲基苯酚 2,5-Dimethylphenol 95-87-4 C8H10O 122.167 4-甲基间苯二酚 4-methyl resorcinol 496-73-1 C7H8O2 124.139 2,4-二甲基苯酚 2,4-Xylenol 105-67-9 C8H10O 122.167 对甲酚 p-cresol 106-44-5 C7H8O 108.14 2-甲基苯甲醚 2-methylmethoxybenzene 578-58-5 C8H10O 122.167 水杨醛 salicylaldehyde 90-02-8 C7H6O2 122.123 2,4,6-三甲酚 Mesitol 527-60-6 C9H12O 136.194 邻羟基苄基溴 o-hydroxybenzyl bromide 58402-38-3 C7H7BrO 187.036 间甲酚 3-methyl-phenol 108-39-4 C7H8O 108.14 水杨醇 salicylic alcohol 90-01-7 C7H8O2 124.139 邻羟基苯甲腈 2-Cyanophenol 611-20-1 C7H5NO 119.123 二甲酚 2,3-Dimethylphenol 526-75-0 C8H10O 122.167 —— O-(2-methylphenyl)hydroxylamine 65440-83-7 C7H9NO 123.155 3-甲基苯邻二酚 3-methylbenzene-1,2-diol 488-17-5 C7H8O2 124.139 4-氯-2-甲基苯酚 2-methyl-4-chlorophenol 1570-64-5 C7H7ClO 142.585 5-碘-2-甲基苯酚 3-hydroxy-4-methyliodobenzene 183803-06-7 C7H7IO 234.036 5-溴-2-甲基苯酚 5-bromo-2-methylphenol 36138-76-8 C7H7BrO 187.036 4-碘-2-甲基苯酚 4-iodo-2-methyl-phenol 60577-30-2 C7H7IO 234.036 2-甲基-4-氨基苯酚 4-amino-2-methylphenol 2835-96-3 C7H9NO 123.155 3,5-二甲基苯酚 3,5-Dimethylphenol 108-68-9 C8H10O 122.167 4-溴-2-甲基苯酚 4-Bromo-2-methylphenol 2362-12-1 C7H7BrO 187.036 4-氘代-2-甲基苯酚 4-deutero-2-methylphenol 1044940-70-6 C7H8O 109.132 4-氟-2-甲基苯酚 4-fluoro-2-methylphenol 452-72-2 C7H7FO 126.13 4-羟-3-甲基苯硫酚 4-mercapto-2-methylphenol 32281-01-9 C7H8OS 140.206 2-甲基苯基甲酸酯 2-methylphenyl formate 1864-95-5 C8H8O2 136.15 —— 1-vinyloxy-2-methylbenzene 934-21-4 C9H10O 134.178 邻乙氧基甲苯 2-ethoxytoluene 614-71-1 C9H12O 136.194 3-甲基-4-羟基苯甲醛 4-hydroxy-3-methyl-benzaldehyde 15174-69-3 C8H8O2 136.15 水杨醛肟 salicylaldehyde-oxime 94-67-7 C7H7NO2 137.138 —— 1-(ethynyloxy)-2-methylbenzene 21368-69-4 C9H8O 132.162 2-甲基-4-甲氧基苯酚 1-hydroxy-4-methoxy-2-methylbenzene 5307-05-1 C8H10O2 138.166 4-羟基-3-甲基苄醇 4-(hydroxymethyl)-2-methylphenol 18299-15-5 C8H10O2 138.166 3-甲基-4-羟基-苯甲腈 4-hydroxy-3-methylbenzonitrile 15777-70-5 C8H7NO 133.15 4-乙基-2-甲基苯酚 4-ethyl-2-methylphenol 2219-73-0 C9H12O 136.194 —— 2-methyl-4-vinylphenol 45803-83-6 C9H10O 134.178 2-氯-6-甲酚 2-Chloro-6-methylphenol 87-64-9 C7H7ClO 142.585 2-羟基-4-甲基苯甲醛 4-methylsalicylaldehyde 698-27-1 C8H8O2 136.15 2-羟基-3-甲基苯甲醛 2-methoxy-3-methylbenzaldehyde 824-42-0 C8H8O2 136.15 3-甲基水杨醇 3-methylsalicyl alcohol 22470-99-1 C8H10O2 138.166 2-羟基-3-甲苯甲腈 2-hydroxy-3-methylbenzonitrile 13589-71-4 C8H7NO 133.15 —— 6-iodo-2-methyl-phenol 24885-45-8 C7H7IO 234.036 3-溴-2-甲基苯酚 3-bromo-2-methylphenol 7766-23-6 C7H7BrO 187.036 2-乙基-6-甲基苯酚 2-ethyl-6-methylphenol 1687-64-5 C9H12O 136.194 2,3,4-三甲基苯酚 2,3,4-trimethylphenol 526-85-2 C9H12O 136.194 苯酚,2-乙烯基-6-甲基- 2-methyl-6-vinylphenol 135295-09-9 C9H10O 134.178 —— α-Chlor-2,6-xylenol —— C8H9ClO 156.612 6-氨基-2-甲基苯酚 2-amino-6-methylphenol 17672-22-9 C7H9NO 123.155 2-甲基-6-氟苯酚 2-fluoro-6-methylphenol 443-90-3 C7H7FO 126.13 —— 2‐mercapto‐6‐methylphenol 25674-50-4 C7H8OS 140.206 1-甲基-2-(苯氧基)苯 1-methyl-2-phenoxybenzene 3991-61-5 C13H12O 184.238 6-溴-2-甲基苯酚 2-bromo-6-methylphenol 13319-71-6 C7H7BrO 187.036 苯甲基苯酚 o-Benzylphenol 28994-41-4 C13H12O 184.238 - 1
- 2
- 3
- 4
- 5
- 6
反应信息
-
作为反应物:描述:参考文献:名称:DE160304摘要:公开号:
-
作为产物:描述:参考文献:名称:Folkers; Adkins, Journal of the American Chemical Society, 1932, vol. 54, p. 1146摘要:DOI:
-
作为试剂:参考文献:名称:Perkin, Journal of the Chemical Society, 1892, vol. 61, p. 462摘要:DOI:
文献信息
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
[EN] SUBSTITUTED QUINAZOLINES AS FUNGICIDES<br/>[FR] QUINAZOLINES SUBSTITUÉES, UTILISÉES EN TANT QUE FONGICIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2010136475A1公开(公告)日:2010-12-02The present invention relates to a compound of formula (I) wherein wherein the substituents have the definitions as defined in claim 1or a salt or a N-oxide thereof, their use and methods for the control and/or prevention of microbial infection, particularly fungal infection, in plants and to processes for the preparation of these compounds.本发明涉及一种具有如下式(I)的化合物,其中取代基具有权利要求1中定义的定义,或其盐或N-氧化物,它们的用途以及用于控制和/或预防植物中微生物感染,特别是真菌感染的方法,以及制备这些化合物的方法。
-
[EN] CALPAIN MODULATORS AND THERAPEUTIC USES THEREOF<br/>[FR] MODULATEURS DE CALPAÏNE ET LEURS UTILISATIONS THÉRAPEUTIQUES申请人:BLADE THERAPEUTICS INC公开号:WO2019190885A1公开(公告)日:2019-10-03Small molecule calpain modulator compounds, including their pharmaceutically acceptable salts, can be included in pharmaceutical compositions. The compounds can be useful in inhibiting calpain, or competitive binding with calpastatin, by contacting them with CAPN1, CAPN2, and/or CAPN9 enzymes residing inside a subject. The compounds and composition can also be administered to a subject in order to treat a fibrotic disease or a secondary disease state or condition of a fibrotic disease.
-
Dual Pharmacophores - PDE4-Muscarinic Antagonistics申请人:Callahan James Francis公开号:US20090203657A1公开(公告)日:2009-08-13The present invention is directed to novel compounds of Formula (I) and pharmaceutically acceptable salts thereof, pharmaceutical compositions and their use as dual chromaphores having inhibitory activity against PDE4 and muscarinic acetylcholine receptors (mAChRs), and thus being useful for treating respiratory diseases.本发明涉及具有式(I)的新化合物及其药用盐,药物组合物及其用作对PDE4和肌胆碱受体(mAChRs)具有抑制活性的双色素,因此可用于治疗呼吸道疾病。
-
Transition metal complexes in organic synthesis, part 43. First total synthesis of the free radical scavenger (±)-neocarazostatin B via iron- and nickel-mediated coupling reactions作者:Hans-Joachim Knölker、Wolfgang Fröhner、Alfred WagnerDOI:10.1016/s0040-4039(98)00527-9日期:1998.5The first total synthesis of the naturally occurring free radical scavenger (±)-neocarazostatin B is described by using a one-pot iron-mediated construction of the carbazole skeleton and a nickle-mediated prenylation as the key-steps.
表征谱图
-
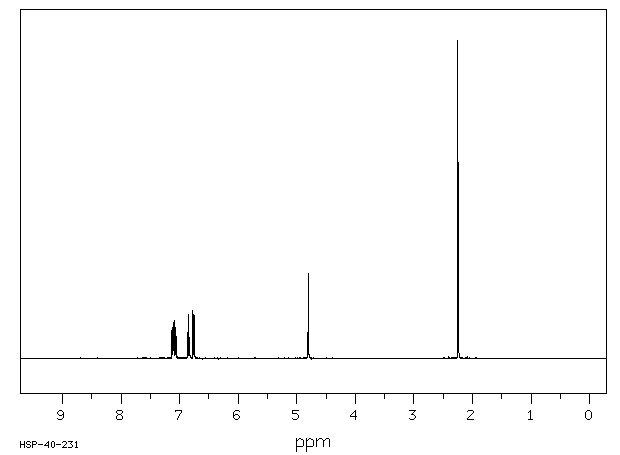
氢谱1HNMR
-
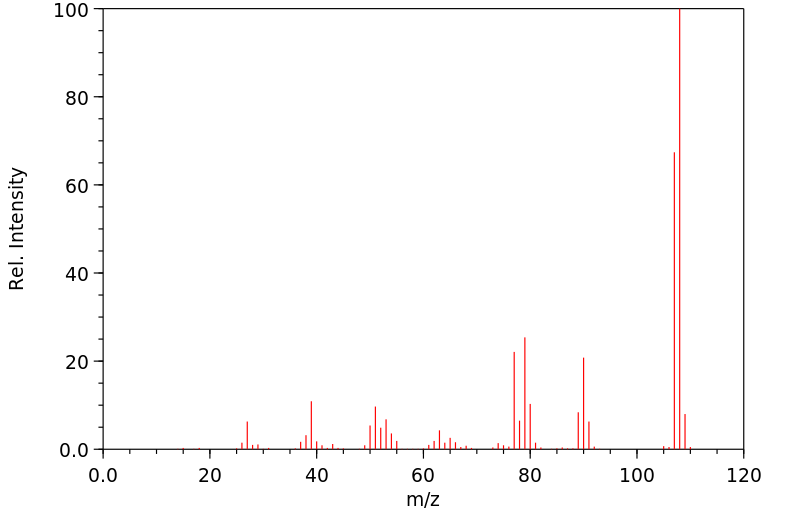
质谱MS
-
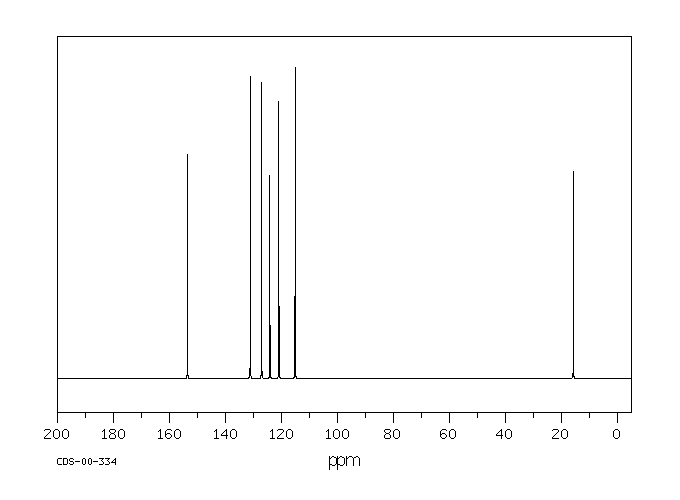
碳谱13CNMR
-
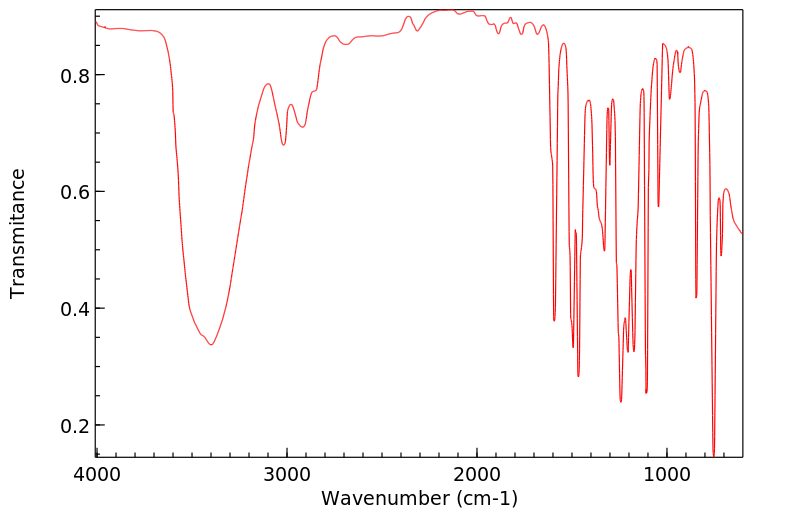
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息