对溴甲基苯甲酸 | 6232-88-8
物质功能分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:224-229 °C(lit.)
-
沸点:252.95°C (rough estimate)
-
密度:1.5313 (rough estimate)
-
稳定性/保质期:
常温常压下稳定,白灰以片状结晶形式存在。熔点为227.5-229℃。
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险等级:8
-
安全说明:S26,S36
-
危险品运输编号:3261
-
WGK Germany:3
-
海关编码:29163900
-
危险类别:8
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
包装等级:III
-
储存条件:请将药品存放在避光、阴凉干燥处,并密封保存。
SDS
模块 1. 化学品
1.1 产品标识符
: 4-(溴甲基)苯甲酸
产品名称
1.2 鉴别的其他方法
α-Bromo-p-toluic acid
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
皮肤刺激 (类别 2)
眼睛刺激 (类别 2A)
特异性靶器官系统毒性(一次接触) (类别 3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防措施
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
事故响应
P302 + P352 如果皮肤接触:用大量肥皂和水清洗。
P304 + P340 如吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P312 如感觉不适,呼救中毒控制中心或医生.
P321 具体处置(见本标签上提供的急救指导)。
P332 + P313 如觉皮肤刺激:求医/就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。
P362 脱掉沾污的衣服,清洗后方可再用。
安全储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物
催泪
模块 3. 成分/组成信息
3.1 物 质
: α-Bromo-p-toluic acid
别名
: C8H7BrO2
分子式
: 215.04 g/mol
分子量
组分 浓度或浓度范围
α-Bromotoluic acid
<=100%
化学文摘登记号(CAS 6232-88-8
No.) 228-343-3
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
取决于接触的时间和强度。程度从轻度刺激到严重组织损伤不等。,
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 溴化氢气
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。
人员疏散到安全区域。 避免吸入粉尘。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
暴露在潮湿中。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
飞溅保护
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 224 - 229 °C
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
碱, 胺, 氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
取决于接触的时间和强度。程度从轻度刺激到严重组织损伤不等。,
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
对溴甲基苯甲酸是一种苯甲酸类化合物,是重要的结构单元之一,能够转化为多种其他重要的有机化合物。
合成方法 步骤 S1将镁粉、碘粉和四氢呋喃加入反应釜中,并使用氮气进行保护。在转速为 100r/min 和温度为 155℃ 的条件下滴加对溴甲苯,进行回流反应 1h 后,通入二氧化碳至充满反应釜,在温度为 3℃ 的条件下继续反应 30min。加入盐酸溶液,进行反应 1h,制得对甲基苯甲酸。
步骤 S2将步骤 S1 制得的对甲基苯甲酸、N-溴代琥珀酰亚胺和四氯化碳加入反应釜中,在转速为 200r/min 的条件下搅拌至对甲基苯甲酸和 N-溴代琥珀酰亚胺完全溶解后,加入过氧化二苯甲酰。在温度为 80℃ 的条件下进行回流反应 2h,制得对溴甲基苯甲酸。
化学性质白灰片状结晶,熔点为 227.5-229℃。
制备方法 用途主要用于医药中间体及有机合成原料等。
生产方法上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对甲基苯甲酸 p-Toluic acid 99-94-5 C8H8O2 136.15 4-(溴甲基)苯甲酸乙酯 4-carbethoxybenzyl bromide 26496-94-6 C10H11BrO2 243.1 苯甲酸 benzoic acid 65-85-0 C7H6O2 122.123 对溴甲基苯甲酰溴 4-(bromomethyl)benzoyl bromide 876-07-3 C8H6Br2O 277.943 对甲基苯甲酸乙酯 4-methylbenzoic acid ethyl ester 94-08-6 C10H12O2 164.204 3-溴甲基苯甲酸 3-bromomethylbenzoic acid 6515-58-8 C8H7BrO2 215.046 4-溴甲基苯甲酰氯 4-bromomethylbenzoyl chloride 52780-16-2 C8H6BrClO 233.492 间甲基苯甲酸 m-Toluic acid 99-04-7 C8H8O2 136.15 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-溴甲基苯甲酸甲酯 Methyl 4-(bromomethyl)benzoate 2417-72-3 C9H9BrO2 229.073 对甲基苯甲酸 p-Toluic acid 99-94-5 C8H8O2 136.15 对醛基苯甲酸 4-Carboxybenzaldehyde 619-66-9 C8H6O3 150.134 对苯二甲酸 phthalic acid 100-21-0 C8H6O4 166.133 4-羟甲基苯甲酸 3-hydroxymethyl-benzoic acid 3006-96-0 C8H8O3 152.15 4-(溴甲基)苯甲酸乙酯 4-carbethoxybenzyl bromide 26496-94-6 C10H11BrO2 243.1 —— isopropyl 4-(bromomethyl)benzoate 28188-39-8 C11H13BrO2 257.127 —— allyl-4-(bromomethyl)benzoate 155030-52-7 C11H11BrO2 255.111 4-甲氧基甲基苯甲酸 4-(Methoxymethyl)benzoic acid 67003-50-3 C9H10O3 166.177 4-溴甲基苯甲醛 4-(bromomethyl)benzaldehyde 51359-78-5 C8H7BrO 199.047 苄基4-(溴甲基)苯甲酸酯 benzyl α-bromo-p-toluate 113100-79-1 C15H13BrO2 305.171 4-溴 甲基苄基醇 4-(bromomethyl)benzyl alcohol 71831-21-5 C8H9BrO 201.063 4-乙烯基苯甲酸 4-Vinylbenzoic acid 1075-49-6 C9H8O2 148.161 4-溴甲基苯甲酸叔丁酯 4-(bromomethyl)benzoic acid 1,1-dimethylethyl ester 108052-76-2 C12H15BrO2 271.154 甲基 4-(甲氧基甲基)苯酸酯 methyl 4-(methoxymethyl)benzoate 1719-82-0 C10H12O3 180.203 4-疏基甲基苯甲酸 4-mercaptomethylbenzoic acid 39088-65-8 C8H8O2S 168.216 对氯甲基苯甲酸 p-(chloromethyl)benzoic acid 1642-81-5 C8H7ClO2 170.595 —— 4-Bromomethylbenzoic acid r-butyl ester 86582-55-0 C12H15BrO2 271.154 4-氨甲基苯甲酸 p-aminomethylbenzoic acid 56-91-7 C8H9NO2 151.165 —— [4-(Bromomethyl)benzoyl] 4-(bromomethyl)benzoate 207114-27-0 C16H12Br2O3 412.077 —— 4-(fluoromethyl)benzoic acid 118507-45-2 C8H7FO2 154.141 —— 4-(iodomethyl)benzoic acid 31719-79-6 C8H7IO2 262.047 4-乙氧基甲基苯甲酸 4-(ethoxymethyl)benzoic acid 146781-28-4 C10H12O3 180.203 —— p-Benzyloxymethyl-benzoesaeure 22714-44-9 C15H14O3 242.274 —— 2-Trimethylsilylethyl 4-(bromomethyl)benzoate 191987-14-1 C13H19BrO2Si 315.282 4-异丙氧基甲基-苯甲酸 4-isopropoxymethylbenzoic acid 850021-34-0 C11H14O3 194.23 对溴甲基苯甲酰溴 4-(bromomethyl)benzoyl bromide 876-07-3 C8H6Br2O 277.943 烯丙基-4-羧基苄基醚 Allyl 4-carboxybenzyl ether 1061592-33-3 C11H12O3 192.214 十六烷基4-(溴甲基)苯甲酸酯 hexadecyl 4-(bromomethyl)benzoate 920982-13-4 C24H39BrO2 439.476 —— 4-[(S)-2-methylbutoxycarbonyl]benzylbromide 83102-77-6 C13H17BrO2 285.181 —— R-2'-methylbutyl-(4-bromomethyl) benzoate 124770-51-0 C13H17BrO2 285.181 4-((2-甲氧基乙氧基)甲基)苯甲酸 4-(2-Methoxy-ethoxymethyl)-benzoic acid 119828-60-3 C11H14O4 210.23 —— 4-ethoxymethyl-benzoic acid ethyl ester 133017-02-4 C12H16O3 208.257 4-(甲基氨基甲基)苯甲酸 4-<(methylamino)methyl>benzoic acid 96084-38-7 C9H11NO2 165.192 4-(氰甲基)苯甲酸 4-(cyanomethyl)benzoic acid 50685-26-2 C9H7NO2 161.16 4-乙烯基苯甲酸甲酯 methyl 4-vinylbenzoate 1076-96-6 C10H10O2 162.188 —— 4,4'-(ethene-1,2-diyl)dibenzoic acid 793-07-7 C16H14O4 270.285 4-丁-3-烯氧基甲基-苯甲酸 4-but-3-enyloxymethyl-benzoic acid 1037159-35-5 C12H14O3 206.241 4-乙酰氧基甲基苯甲酸 p-acetoxymethyl benzoic acid 15561-46-3 C10H10O4 194.187 4,4'-[(E)-1,2-二氮烯二基二(氧基亚甲基)]二苯甲酸 bis(4-carboxybenzyl)hyponitrite 223507-96-8 C16H14N2O6 330.297 4-(3-丁烯-1-基)苯甲酸 4-(but-3-en-1-yl)benzoic acid 15451-35-1 C11H12O2 176.215 —— nonaethyleneglycol(4-carboxybenzyl) methyl ether 259226-87-4 C27H46O12 562.655 4-(叠氮甲基)苯甲酸 4-(azidomethyl)benzoic acid 79584-03-5 C8H7N3O2 177.162 —— 4-[(nitrooxy)methyl]benzoic acid 258278-55-6 C8H7NO5 197.147 —— 4-Bromomethyl benzoic acid phenyl ester 35092-35-4 C14H11BrO2 291.144 对氰基甲基苯甲酸甲酯 methyl 4-(cyanomethyl)benzoate 76469-88-0 C10H9NO2 175.187 4-溴甲基苯甲酰氯 4-bromomethylbenzoyl chloride 52780-16-2 C8H6BrClO 233.492 —— 4-(propylthiomethyl)benzoic acid 1019561-88-6 C11H14O2S 210.297 —— 4‐(((2‐hydroxyethyl)thio)methyl)benzoic acid —— C10H12O3S 212.269 4-溴甲基苯甲酰胺 4-(bromomethyl)benzamide 58914-40-2 C8H8BrNO 214.062 —— 1-bromomethyl-4-(tert-butyloxymethyl)benzene 939378-19-5 C12H17BrO 257.17 —— (2-octyldodecyl) 4-bromomethylbenzoate 808142-53-2 C28H47BrO2 495.584 升对酞酸 4-(carboxymethyl)benzoic acid 501-89-3 C9H8O4 180.16 —— 4-(2-oxopropyl)benzoic acid 15482-54-9 C10H10O3 178.188 —— α-carbamoyl-p-toluic acid 52787-17-4 C9H9NO3 179.175 —— 4-(3-oxobutyl)benzoic acid 74248-66-1 C11H12O3 192.214 - 1
- 2
- 3
- 4
- 5
- 6
反应信息
-
作为反应物:参考文献:名称:Triflylpyridinium 能够使用 Pinacolborane 快速且可扩展地将羧酸控制还原为醛摘要:通过使用频哪醇硼烷作为还原剂和三氟甲基吡啶作为活化剂,实现了从羧酸中快速且可扩展地合成醛类。理论研究表明,酰基吡啶的还原需要比产物醛更低的活化自由能。该方法具有广泛的底物适用范围。实现了流动合成和氘代醛的合成。DOI:10.1002/anie.202215168
-
作为产物:描述:参考文献:名称:Selective Hydrogenolysis of Bis(hydroxymethyl)aromatic Compounds摘要:在碱性溶液中,采用Raney镍对对称双(羟甲基)芳香化合物进行选择性氢解,温度为20°C,时间为24至36小时。对应单氢解产物的产率在75%至96%之间。通过合成4-(溴甲基)苯甲酸,展示了该反应的实用性。DOI:10.1055/s-1992-26346
-
作为试剂:参考文献:名称:CYCLOPROPYLAMINES AS LSD1 INHIBITORS摘要:本发明涉及使用环丙胺衍生物来调节,特别是抑制赖氨酸特异性去甲基化酶1(LSD1)的活性。适当地,本发明涉及使用环丙胺衍生物治疗癌症。公开号:US20140371176A1
文献信息
-
Fused imidazoles as potential chemical scaffolds for inhibition of heat shock protein 70 and induction of apoptosis. Synthesis and biological evaluation of phenanthro[9,10-d]imidazoles and imidazo[4,5-f][1,10]phenanthrolines
-
Investigations into the Potential Role of Metabolites on the Anti-Leukemic Activity of Imatinib, Nilotinib and Midostaurin作者:Paul W. ManleyDOI:10.2533/chimia.2019.561日期:——
The efficacy and side-effects of drugs do not just reflect the biochemical and pharmacodynamic properties of the parent compound, but often comprise of cooperative effects between the properties of the parent and active metabolites. Metabolites of imatinib, nilotinib and midostaurin have been synthesised and evaluated in assays to compare their properties as protein kinase inhibitors with the parent drugs. The N-desmethyl-metabolite of imatinib is substantially less active than imatinib as a BCR-ABL1 kinase inhibitor, thus providing an explanation as to why patients producing high levels of this metabolite show a relatively low response rate in chronic myeloid leukaemia (CML) treatment. The hydroxymethylphenyl and N-oxide metabolites of imatinib and nilotinib are only weakly active as BCR-ABL1 inhibitors and are unlikely to play a role in the efficacy of either drug in CML. The 3-(R)-HO-metabolite of midostaurin shows appreciable accumulation following chronic drug administration and, in addition to mutant forms of FLT3, potently inhibits the PDPK1 and VEGFR2 kinases (IC50 values
药物的功效和副作用不仅仅反映了母化合物的生化和药效特性,而且通常包括母化合物和活性代谢物之间的协同效应。已经合成和评估了伊马替尼、尼洛替尼和米多斯他林的代谢物,以比较它们作为蛋白激酶抑制剂的特性与母药的区别。伊马替尼的N-去甲基代谢物作为BCR-ABL1激酶抑制剂的活性明显低于伊马替尼,这解释了为什么产生高水平该代谢物的患者在慢性髓细胞白血病(CML)治疗中显示相对较低的反应率。伊马替尼和尼洛替尼的羟甲基苯和N-氧代谢物作为BCR-ABL1抑制剂的活性很弱,不太可能在CML中发挥作用。米多斯他林的3-(R)-HO-代谢物在长期用药后显示出明显的积累,并且除了FLT3的突变形式外,还强力抑制PDPK1和VEGFR2激酶(IC50值 -
Potential antiatherosclerotic agents. 3. Substituted benzoic and nonbenzoic acid analogs of cetaben作者:J. Donald Albright、Vern G. DeVries、Mila T. Du、Elwood E. Largis、Thomas G. Miner、Marvin F. Reich、Robert G. ShepherdDOI:10.1021/jm00364a010日期:1983.10acid group of cetaben is replaced by carboxylate ester, carboxamide, or a variety of other substituent groups is described. Also reported are the syntheses of analogues in which the phenyl ring of cetaben is either modified by the presence of additional substituents or replaced entirely by another moiety. Structure-activity relationships of these compounds both as hypolipidemic agents and as inhibitors
-
FLAP MODULATORS申请人:JANSSEN PHARMACEUTICA NV公开号:US20140221310A1公开(公告)日:2014-08-07The present invention relates to compounds of Formula (I), or a form thereof, wherein ring A, R 1 , L and R 2 are as defined herein, useful as FLAP modulators. The invention also relates to pharmaceutical compositions comprising compounds of Formula (I). Methods of making and using the compounds of Formula (I) are also within the scope of the invention.本发明涉及式(I)化合物,或其形式,其中环A,R1,L和R2如本文所定义,作为FLAP调节剂有用。该发明还涉及包含式(I)化合物的药物组合物。制备和使用式(I)化合物的方法也属于本发明的范围。
-
N,N-Di-alkyl(phenoxy)benzamide derivatives申请人:G. D. Searle & Co.公开号:US05223539A1公开(公告)日:1993-06-29The present invention relates to compounds of the formula: ##STR1## and the pharmaceutically acceptable salts thereof, wherein Z can be: ##STR2## wherein R.sup.3 is alkyl having 1 to 6 carbon atoms and, when n is greater than 1, each R.sup.3 can be the same or different; and n is an integer from 1 to 3; R.sup.1 and R.sup.2 can each independently be hydrogen, straight or branched chain alkyl, or cycloalkyl having 3 to 8 carbon atoms which can optionally be substituted at one or more positions by alkyl of 1 to 6 carbon atoms; X is oxygen, sulfur, NR.sup.4, wherein R.sup.4 is hydrogen or alkyl having 1 to 4 carbon atoms, C.dbd.O, CHOH, or CH.sub.2 ; Y is hydrogen, alkoxy, halogen, alkyl, or hydroxy; and m is an integer from 0 to 3. The compounds are antagonists of platlet-activating factor (PAF).本发明涉及以下式的化合物:##STR1##及其药学上可接受的盐,其中Z可以是:##STR2##其中R.sup.3是具有1至6个碳原子的烷基,当n大于1时,每个R.sup.3可以相同或不同;n是1至3之间的整数;R.sup.1和R.sup.2可以分别是氢、直链或支链烷基,或者是具有3至8个碳原子的环烷基,可以在一个或多个位置被1至6个碳原子的烷基取代;X是氧、硫、NR.sup.4,其中R.sup.4是氢或具有1至4个碳原子的烷基,C.dbd.O,CHOH或CH.sub.2;Y是氢、烷氧基、卤素、烷基或羟基;m是0至3之间的整数。这些化合物是血小板活化因子(PAF)的拮抗剂。
表征谱图
-
氢谱1HNMR
-
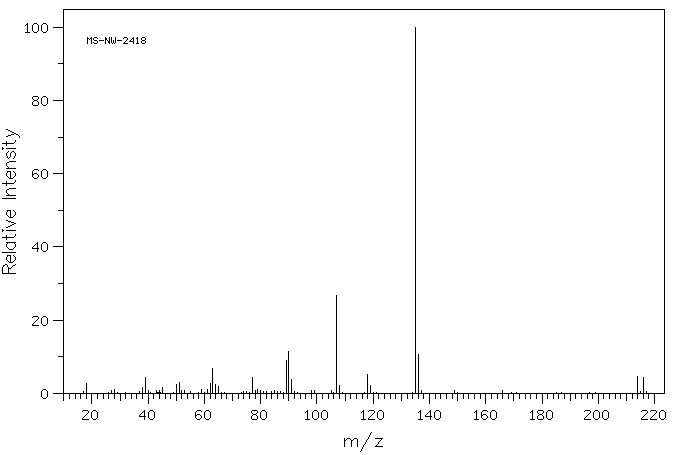
质谱MS
-
碳谱13CNMR
-
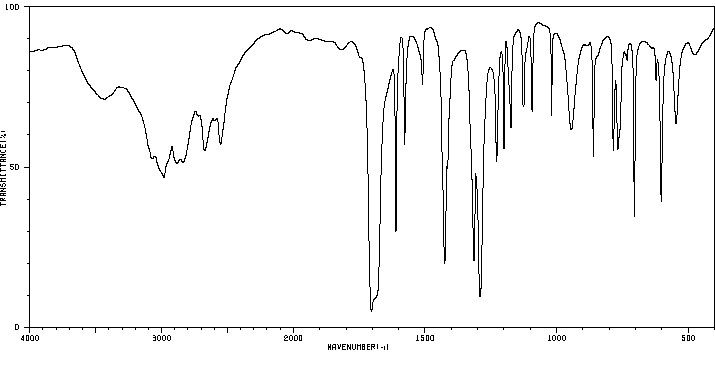
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息