对苯二甲醇 | 589-29-7
中文名称
对苯二甲醇
中文别名
对二甲苯-α,α'-二醇;1,4-苯二甲醇
英文名称
p-xylylene glycol
英文别名
1,4-Benzenedimethanol;1,4-phenylenedimethanol;terephthalyl alcohol;1,4-di(hydroxymethyl)benzene;[4-(hydroxymethyl)phenyl]methanol
CAS
589-29-7
化学式
C8H10O2
mdl
——
分子量
138.166
InChiKey
BWVAOONFBYYRHY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:114-118 °C(lit.)
-
沸点:138-143 °C1 mm Hg(lit.)
-
密度:1.0742 (rough estimate)
-
闪点:138-143°C/1mm
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:10
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险品标志:Xn
-
安全说明:S22,S23,S24/25,S7,S9
-
危险类别码:R20/22
-
WGK Germany:3
-
海关编码:29062900
-
危险品运输编号:NONH for all modes of transport
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302+H312+H332,H315,H401
-
储存条件:室温
SDS
1.1 产品标识符
: 1,4-Benzenedimethanol
化学品俗名或商品名
1.2 鉴别的其他方法
p-Xylylene dialcohol
p-Xylene-α,α′-diol
p-Phenylene dicarbinol
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: p-Xylylene dialcohol
别名
p-Xylene-α,α′-diol
p-Phenylene dicarbinol
: C8H10O2
分子式
: 138.16 g/mol
分子量
成分 浓度
p-Phenylenedimethanol
-
化学文摘编号(CAS No.) 589-29-7
EC-编号 209-641-2
模块 4. 急救措施
4.1 必要的急救措施描述
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
在皮肤接触的情况下
用肥皂和大量的水冲洗。
在眼睛接触的情况下
用水冲洗眼睛作为预防措施。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 最重要的症状和影响,急性的和滞后的
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止粉尘的生成。 防止吸入蒸汽、气雾或气体。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
扫掉和铲掉。 存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
人身保护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所来选择人体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 结晶
颜色: 棕灰色
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/熔点范围: 114 - 118 °C - lit.
f) 起始沸点和沸程
138 - 143 °C 在 1 hPa - lit.
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
酸, 酰氯, 酸酐, 氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
上述信息视为正确,但不包含所有的信息,仅作为指引使用。本文件中的信息是基于我们目前所知,就正
确的安全提示来说适用于本品。该信息不代表保证此产品的性质。
公司对任何操作或者接触上述产品而引起的损害不负有任何责任,。更多使用条款,参见发票或包
装条的反面。
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
: 1,4-Benzenedimethanol
化学品俗名或商品名
1.2 鉴别的其他方法
p-Xylylene dialcohol
p-Xylene-α,α′-diol
p-Phenylene dicarbinol
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: p-Xylylene dialcohol
别名
p-Xylene-α,α′-diol
p-Phenylene dicarbinol
: C8H10O2
分子式
: 138.16 g/mol
分子量
成分 浓度
p-Phenylenedimethanol
-
化学文摘编号(CAS No.) 589-29-7
EC-编号 209-641-2
模块 4. 急救措施
4.1 必要的急救措施描述
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
在皮肤接触的情况下
用肥皂和大量的水冲洗。
在眼睛接触的情况下
用水冲洗眼睛作为预防措施。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 最重要的症状和影响,急性的和滞后的
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止粉尘的生成。 防止吸入蒸汽、气雾或气体。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
扫掉和铲掉。 存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
人身保护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所来选择人体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 结晶
颜色: 棕灰色
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/熔点范围: 114 - 118 °C - lit.
f) 起始沸点和沸程
138 - 143 °C 在 1 hPa - lit.
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
酸, 酰氯, 酸酐, 氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
上述信息视为正确,但不包含所有的信息,仅作为指引使用。本文件中的信息是基于我们目前所知,就正
确的安全提示来说适用于本品。该信息不代表保证此产品的性质。
公司对任何操作或者接触上述产品而引起的损害不负有任何责任,。更多使用条款,参见发票或包
装条的反面。
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 (4-甲基苯基)甲醇 4-Methylbenzyl alcohol 589-18-4 C8H10O 122.167 对羟基甲基苯甲醛 4-(hydroxylmethyl)benzaldehyde 52010-97-6 C8H8O2 136.15 1,4-双(异丙氧基甲基)苯 1,4-bis(isopropoxymethyl)benzene 100079-29-6 C14H22O2 222.327 4-羟甲基苯甲酸 3-hydroxymethyl-benzoic acid 3006-96-0 C8H8O3 152.15 对苯二甲酸 phthalic acid 100-21-0 C8H6O4 166.133 对二甲苯 para-xylene 106-42-3 C8H10 106.167 对醛基苯甲酸 4-Carboxybenzaldehyde 619-66-9 C8H6O3 150.134 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (4-甲基苯基)甲醇 4-Methylbenzyl alcohol 589-18-4 C8H10O 122.167 苯甲醇 benzyl alcohol 100-51-6 C7H8O 108.14 (4-甲氧基甲基苯基)甲醇 [4-(methoxymethyl)phenyl]methanol 62172-89-8 C9H12O2 152.193 对苯二甲基二甲醚 1,4-dimethoxymethyl benzene 6770-38-3 C10H14O2 166.22 对羟基甲基苯甲醛 4-(hydroxylmethyl)benzaldehyde 52010-97-6 C8H8O2 136.15 —— (4-vinyloxymethyl-phenyl)-methanol 391243-83-7 C10H12O2 164.204 —— bis-vinyl ether 193687-66-0 C12H14O2 190.242 4-溴 甲基苄基醇 4-(bromomethyl)benzyl alcohol 71831-21-5 C8H9BrO 201.063 4-(氯甲基)苯甲基醇 4-(hydroxymethyl)benzyl chloride 16473-35-1 C8H9ClO 156.612 —— 1,4-phenylenebis(methylene) diformate 71190-73-3 C10H10O4 194.187 —— (4-(phenylmethoxymethyl)phenyl)methanol 934667-37-5 C15H16O2 228.291 —— 1,4-bis-benzyloxymethyl-benzene 16721-03-2 C22H22O2 318.415 —— 1,4-bis(ethoxymethyl)benzene 112039-24-4 C12H18O2 194.274 —— (4-methoxymethoxymethylphenyl)-methanol 207223-84-5 C10H14O3 182.219 —— 2,4,13,15-tetraoxa[5,5]paracyclophane —— C18H20O4 300.354 —— 2,4,13,15,24,26-hexaoxa[5,5,5]paracyclophane —— C27H30O6 450.532 —— 2,4,13,15,24,26,35,37-octaoxa[5,5,5,5]paracyclophane —— C36H40O8 600.709 —— (4-((2-fluoroethoxy)methyl)phenyl)methanol —— C10H13FO2 184.21 —— 1,4-bis[(allyloxy)methyl]benzene 26332-23-0 C14H18O2 218.296 4-羟甲基苯甲酸 3-hydroxymethyl-benzoic acid 3006-96-0 C8H8O3 152.15 —— 4-[2-propynylmethoxy]benzyl alcohol —— C11H12O2 176.215 —— 1,4-bis(prop-2-ynyloxymethyl)benzene 18473-19-3 C14H14O2 214.264 对苯二甲酸 phthalic acid 100-21-0 C8H6O4 166.133 对二甲苯 para-xylene 106-42-3 C8H10 106.167 —— 1-(bromomethyl)-4-(methoxymethyl)benzene 95349-71-6 C9H11BrO 215.09 对醛基苯甲酸 4-Carboxybenzaldehyde 619-66-9 C8H6O3 150.134 —— p-Di-(tert.-butoxymethyl)-benzol 62667-43-0 C16H26O2 250.381 邻苯二甲醇 phthalyl alcohol 612-14-6 C8H10O2 138.166 —— 1,4-bis(trimethylsilyloxymethyl)benzene 2117-22-8 C14H26O2Si2 282.53 —— 1,4-benzenedimethanol monotrimethylsilyl ether —— C11H18O2Si 210.348 —— 1,4-phenylenedimethylene bis-chloroformate 10362-03-5 C10H8Cl2O4 263.077 [4-(羟甲基)苯基]甲醇乙酸酯 1,4-bis(hydroxymethyl)benzene diacetate 14720-70-8 C12H14O4 222.241 —— 4-acetoxymethylbenzyl alcohol 93914-54-6 C10H12O3 180.203 [4-(2,2,2-三氟乙氧基甲基)-苯基]-甲醇 [4-(2,2,2-trifluoroethoxymethyl)-phenyl]-methanol 1058167-56-8 C10H11F3O2 220.191 —— 4-(hydroxymethyl)benzyl nitrate —— C8H9NO4 183.164 (4-(叠氮甲基)苯基)甲醇 [4-(azidomethyl)phenyl]methanol 439691-96-0 C8H9N3O 163.179 —— 4-((Benzyloxy)methyl)benzaldehyde 216384-72-4 C15H14O2 226.275 —— 1-bromomethyl-4-phenylmethoxymethylbenzene 147876-75-3 C15H15BrO 291.187 —— 1,4-Bis(pent-4-enoxymethyl)benzene 503822-27-3 C18H26O2 274.403 —— Bis(4-chlorobutyl)-1,4-xylylene diether 157870-85-4 C16H24Cl2O2 319.271 —— 1,4-Bis(pent-4-ynoxymethyl)benzene 195611-58-6 C18H22O2 270.371 —— [4-[[4-[[4-(Hydroxymethyl)phenyl]methylsulfanylmethyl]phenyl]methylsulfanylmethyl]phenyl]methanol 144852-56-2 C24H26O2S2 410.601 - 1
- 2
- 3
- 4
- 5
反应信息
-
作为反应物:参考文献:名称:A New Receptor Based on Calix[5]arene Analogue摘要:本文介绍了杯[5]芳烃类似物四甲基醚(1)的合成及其结合行为。通过分子动力学-分子力学计算预测,在结合客体分子时,宿主结构会进行调整以适应客体形状。DOI:10.1055/s-1997-3260
-
作为产物:参考文献:名称:PROCESS FOR PRODUCTION OF AROMATIC ALCOHOL OR HETEROCYCLIC AROMATIC ALCOHOL摘要:公开号:EP2657214B1
-
作为试剂:参考文献:名称:钴复杂的大环炔烃的环加成反应:跨环鲍森-汉德反应摘要:Pauson-Khand反应是通过有效的[2 + 2 + 1]二苯甲醚炔烃与烯烃的环加成反应来合成环戊烯酮的有力工具。尽管分子间和分子内的变体是众所周知的,但是该反应的跨环版本是未知的,并且是这项研究的基础。大环烯炔和二烯炔配合物很容易通过钯(II)催化双(乙烯基硼酸酯)的氧化大环化或闭环易位反应,然后与二钴二羰基八羰基配合而合成。这些大环配合物的几种反应方式被发现。除了第一个成功的跨环形波森-汉德反应之外,其他分子间和跨环形环加成反应还包括分子间波森-汉德反应,跨环[4 + 2]环加成反应,分子间[2 + 2 + 2]环加成反应和分子间[2 + 2 +1 + 1]环加成反应。介绍了每个过程的结构和反应要求。DOI:10.1021/acs.joc.7b01369
文献信息
-
重合性単量体、組成物、硬化性組成物および樹脂部材申请人:株式会社トクヤマデンタル公开号:JP2015182962A公开(公告)日:2015-10-22【課題】種々の材料との相溶性の確保が容易かつ(重合収縮率/硬化物の硬度)が小さい(メタ)アクリレート系重合性単量体及びこれを用いた材料を提供すること。【解決手段】一般式(1)で示される重合性単量体並びにこれを用いた組成物、硬化性組成物および樹脂部材。〔Xは2価の基;Y及びZは(メタ)アクロイルオキシ−L−OCHR1−(R1はH又はC1〜6のアルキル基1価の基、Lは主鎖の炭素数2〜50の炭化水素基である)、Ar1及びAr2は芳香族基、o及びpは1〜3の範囲から選択される整数、m及びnは、各々0又は1;ここで、mが0の場合は、YとAr2とが直に結合し、nが0の場合はAr1とAr2とが直に結合する〕【選択図】なし
-
小白菊内酯-苯磺酰基呋咱衍生物及其盐,制备方法和应用
-
Desymmetrization of Cyclic Anhydrides Using Dihydroxy Compounds: Selective Synthesis of Macrocyclic Tetralactones作者:Sengodagounder Muthusamy、Boopathy Gnanaprakasam、Eringathodi SureshDOI:10.1021/ol060509k日期:2006.4.1desymmetrization of cyclic anhydrides is carried out using dihydroxy compounds. A mild route to synthesize fused saturated/unsaturated macrocyclic tetralactones with different ring sizes (20-34) having a wide variety of spacers is described. The structure is confirmed by the representative single-crystal X-ray analysis. The multiple reduction of unsaturated macrocyclic tetralactones is also illustrated. This method[反应:见正文]使用二羟基化合物进行环状酸酐的脱对称化。描述了合成具有不同间隔基的具有不同环尺寸(20-34)的稠合的饱和/不饱和大环四内酯的温和路线。通过代表性的单晶X射线分析确认了该结构。还示出了不饱和大环四内酯的多次还原。该方法温和,选择性和高效,并且可以实现高收率。
-
Asymmetric Allylic Etherification of Vinylethylene Carbonates with Diols via Pd/B Cooperative Catalysis: A Route to Chiral Hemi-Crown Ethers作者:Sardaraz Khan、Hongfang Li、Can Zhao、Xue Wu、Yong Jian ZhangDOI:10.1021/acs.orglett.9b03663日期:2019.12.6carbonates (VECs) with diols has been developed. By using cooperative catalysts of the chiral palladium complex and triethylborane in mild conditions, the process gave monoetherified and bisetherified polyglycol derivatives with tetrasubstituted stereocenters in high yields with complete regioselectivities and high levels of enantio- and diastereoselectivities.
-
Introducing the reversible chemistry of CO<sub>2</sub> with diols mediated by organic superbases into polycarbonate synthesis作者:Yang Chai、Qin Chen、Caijuan Huang、Qiang Zheng、Michael North、Haibo XieDOI:10.1039/d0gc01197e日期:——
The reversible reaction of CO2 with alcohols mediated by organic superbases was firstly developed to be a toolbox for capturing CO2 into polymerizable carbonate monomers applicable for thiol–ene click and ADMET polymerization to produce new libraries of polycarbonates.
表征谱图
-
氢谱1HNMR
-
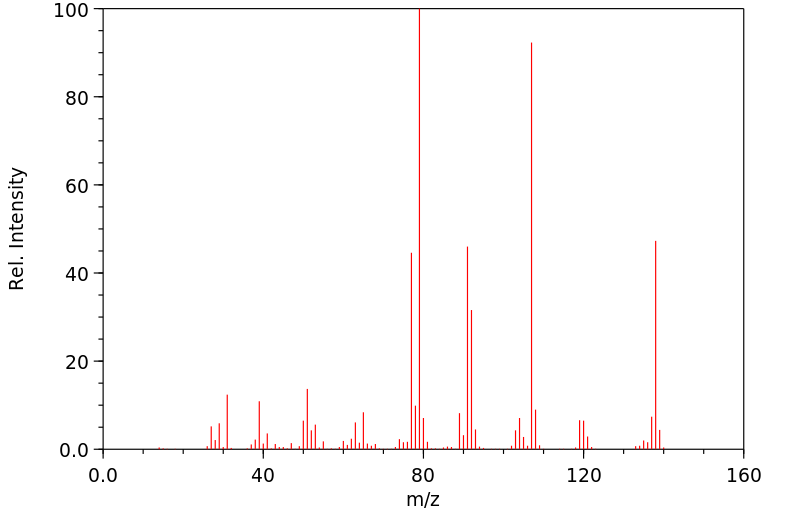
质谱MS
-
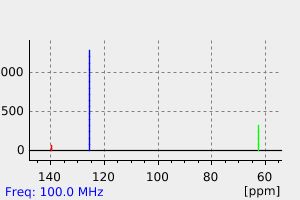
碳谱13CNMR
-
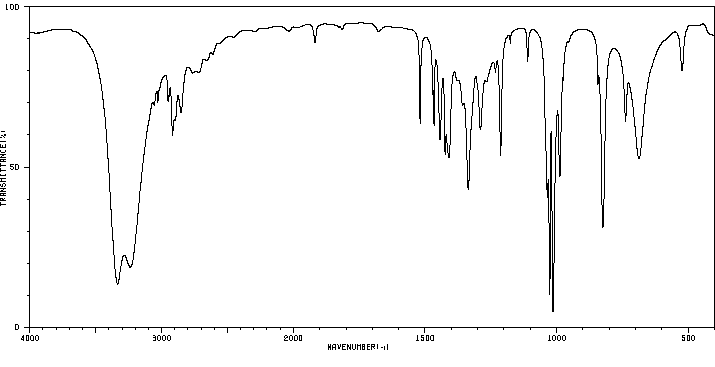
红外IR
-
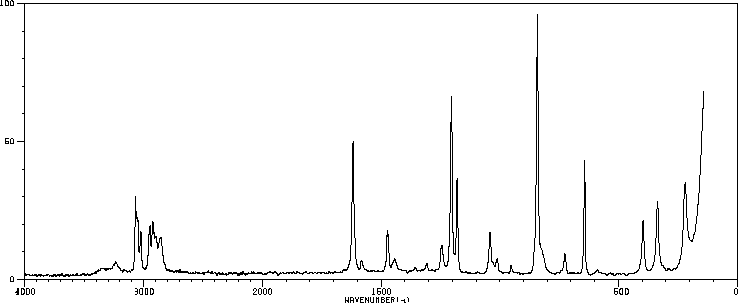
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫