1,4-二氢-4-氧代-3-喹啉羧酸乙酯 | 52980-28-6
中文名称
1,4-二氢-4-氧代-3-喹啉羧酸乙酯
中文别名
4-氧代-1,4-二氢喹啉-3-甲酸乙酯;4-羟基-3-乙氧羰基喹啉;4-羟基-3-喹啉羧酸乙酯;4-氧代-1,4-二氢-3-喹啉甲酸乙酯;3-乙氧羰基-4-羟基喹啉;4-氧代-1,4-二氢喹啉-3-羧酸乙酯;4-氧代-1H-3-喹啉羧酸乙酯;4-氧代-1H-喹啉-3-甲酸乙酯
英文名称
4-oxo-1,4-dihydroquinoline-3-carboxylic acid ethyl ester
英文别名
ethyl 4-oxo-1,4-dihydroquinoline-3-carboxylate;ethyl 4-oxo-1H-quinoline-3-carboxylate
CAS
52980-28-6
化学式
C12H11NO3
mdl
MFCD00173406
分子量
217.224
InChiKey
YBEOYBKKSWUSBR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:270°
-
沸点:343.7±42.0 °C(Predicted)
-
密度:1.246±0.06 g/cm3(Predicted)
-
溶解度:可溶于DMF(轻微加热)、DMSO(轻微加热)
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:16
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.166
-
拓扑面积:55.4
-
氢给体数:1
-
氢受体数:4
安全信息
-
危险等级:IRRITANT
-
海关编码:2933499090
-
危险性防范说明:P261,P280,P301+P312,P302+P352,P305+P351+P338
-
危险性描述:H302,H315,H320,H335
-
储存条件:室温且干燥
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: Ethyl 4-oxo-1,4-dihydro-3-quinolinecarboxylate
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Ethyl 4-oxo-1,4-dihydro-3-quinolinecarboxylate
CAS number: 52980-28-6
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C12H11NO3
Molecular weight: 217.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: Ethyl 4-oxo-1,4-dihydro-3-quinolinecarboxylate
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Ethyl 4-oxo-1,4-dihydro-3-quinolinecarboxylate
CAS number: 52980-28-6
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C12H11NO3
Molecular weight: 217.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-氧代-1,4-二氢喹啉-3-羧酸 4-oxo-1,4-dihydroquinoline-3-carboxylic acid 13721-01-2 C10H7NO3 189.17 4-氯喹啉-3-甲基乙酯 ethyl 4-chloroquinoline-3-carboxylate 13720-94-0 C12H10ClNO2 235.67 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-氧代-1,4-二氢-3-喹啉羧酸甲酯 methyl 4-oxo-1,4-dihydroquinoline-3-carboxylate 61707-79-7 C11H9NO3 203.197 6-氟-4-氧代-1,4-二氢-3-喹啉羧酸乙酯 ethyl 6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate 71083-00-6 C12H10FNO3 235.215 4-羟基-6-三氟甲基喹啉-3-甲酸乙酯 ethyl 4-oxo-6-(trifluoromethyl)-1,4-dihydroquinoline-3-carboxylate 26893-12-9 C13H10F3NO3 285.223 4-氧代-1,4-二氢喹啉-3-羧酸 4-oxo-1,4-dihydroquinoline-3-carboxylic acid 13721-01-2 C10H7NO3 189.17 乙基6,7-二氟-4-氧代-1,4-二氢-3-喹啉羧酸酯 ethyl 6,7-difluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate 121873-01-6 C12H9F2NO3 253.205 1-乙基-1,4-二氢-4-氧代-3-喹啉羧酸乙酯 ethyl 1-ethyl-4-oxo-1,4-dihydroquinoline-3-carboxylate 23789-87-9 C14H15NO3 245.278 —— 4-oxo-1-pentyl-1,4-dihydroquinoline-3-carboxylic acid ethyl ester 875148-74-6 C17H21NO3 287.359 —— 1-hexyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid ethyl ester 875148-75-7 C18H23NO3 301.386 1-甲基-4-氧代-1,4-二氢喹啉-3-羧酸 1,4-dihydro-1-methyl-4-oxoquinoline-3-carboxylic acid 18471-99-3 C11H9NO3 203.197 —— 1-benzyl-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid ethyl ester 53977-02-9 C19H17NO3 307.349 —— 1-(2-phenylethyl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid ethyl ester 23789-91-5 C20H19NO3 321.376 —— 1-(4-chlorobenzyl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid ethyl ester —— C19H16ClNO3 341.794 —— 1-(4-bromobenzyl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid ethyl ester —— C19H16BrNO3 386.245 —— 1-(4-fluorobenzyl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid ethyl ester —— C19H16FNO3 325.339 1-乙基-4-氧代-1,4-二氢喹啉-3-羧酸 1-ethyl-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid 23789-88-0 C12H11NO3 217.224 1-(4-甲氧基苄基)-4-氧代-1,4-二氢喹啉-3-羧酸乙酯 ethyl 1-(4-methoxybenzyl)-4-oxo-1,4-dihydroquinoline-3-carboxylate 937268-26-3 C20H19NO4 337.375 —— 1,3-dicarboethoxy-1,4-dihydroquinolin-4-one 125836-06-8 C15H15NO5 289.288 —— 1-benzyloxycarbonyl-3-carboethoxy-1,4-dihydroquinolin-4-one 125836-08-0 C20H17NO5 351.359 —— 1-n-butyl-1,4-dihydro-4-oxoquinoline-3-carboxylic acid 35975-85-0 C14H15NO3 245.278 4-氧代-1-戊基喹啉-3-羧酸 4-oxo-1-pentyl-1,4-dihydroquinoline-3-carboxylic acid 875148-76-8 C15H17NO3 259.305 —— 1,4-dihydro-1-<(2-hydroxyethoxy)methyl>-4-oxo-3-quinolinecarboxylic acid 143231-28-1 C13H13NO5 263.25 —— 1-benzyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 35975-86-1 C17H13NO3 279.295 —— 1-(4-fluorobenzyl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid —— C17H12FNO3 297.286 —— 1-Oxo-6,7-dihydro-1H,5H-pyrido[3,2,1-ij]quinoline-2-carboxylic acid ethyl ester 59611-46-0 C15H15NO3 257.289 1-(4-甲氧基苄基)-4-氧代-1,4-二氢喹啉-3-羧酸 1-(4-methoxybenzyl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 338747-41-4 C18H15NO4 309.321 —— ethyl 4-methyl-3-quinolinecarboxylate —— C13H13NO2 215.252 4-羟基-喹啉-3-羧酸酰肼 1,4-dihydro-4-oxoquinoline-3-carbohydrazide 103264-46-6 C10H9N3O2 203.2 4-溴-3-喹啉羧酸乙酯 ethyl 4-bromoquinoline-3-carboxylate 1242260-12-3 C12H10BrNO2 280.121 4-氯喹啉-3-甲基乙酯 ethyl 4-chloroquinoline-3-carboxylate 13720-94-0 C12H10ClNO2 235.67 —— 1-(4-(fluorosulfonyl)benzyl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 1613052-75-7 C17H12FNO5S 361.35 —— N-benzyl-4-oxo-1,4-dihydroquinoline-3-carboxamide 943062-05-3 C17H14N2O2 278.31 —— 4-oxo-N'-(4-chlorobenzyl)-1,4-dihydroquinoline-3-carboxamide 228725-38-0 C17H13ClN2O2 312.755 —— 4-Ethynyl-quinoline-3-carboxylic acid ethyl ester 207113-60-8 C14H11NO2 225.247 —— 4-oxo-1,4-dihydroquinoline-3-carboxylic acid phenylamide 857208-58-3 C16H12N2O2 264.283 —— 3-carboethoxy-1-<2',2'-bis-(4-nitrophenyl)ethoxycarbonyl>-1,4-dihydroquinolin-4-one 125836-09-1 C27H21N3O9 531.478 —— 4-hydroxy-N-(4-methoxyphenyl)-3-quinoline-carboxamide —— C17H14N2O3 294.31 —— 4-oxo-N'-(4-fluorophenyl)-1,4-dihydroquinoline-3-carboxamide 1051863-33-2 C16H11FN2O2 282.274 —— penicinoline 1214268-60-6 C14H10N2O3 254.245 —— ethyl 5-oxo-1,2,3,5-tetrahydropyrrolo[1,2-a]quinoline-4-carboxylate 77444-54-3 C15H15NO3 257.289 —— N-cyclohexyl-quinolin-4(1H)-one-3-carboxamide 283166-82-5 C16H18N2O2 270.331 —— 2,3,4,6-Tetrahydro-6-oxo-1H-benzo quinolizine-5-carboxylic Acid Ethyl Ester 77444-55-4 C16H17NO3 271.316 —— 4-Trimethylsilanylethynyl-quinoline-3-carboxylic acid ethyl ester 207113-59-5 C17H19NO2Si 297.429 —— 4-oxo-N'-(2,5-dichlorophenyl)-1,4-dihydroquinoline-3-carboxamide 1091980-94-7 C16H10Cl2N2O2 333.174 —— 3-carboethoxy-1-fluorenylmethoxycarbonyl-1,4-dihydroquinolin-4-one 125867-56-3 C27H21NO5 439.467 —— N-(5-amino-2-methylphenyl)-4-oxo-1H-quinoline-3-carboxamide 873051-10-6 C17H15N3O2 293.325 —— ethyl-1,4-dihydro-4-oxoquinoline-3-carbohydrazide 1403889-01-9 C12H13N3O2 231.254 —— 4-Trimethylsilanyloxy-quinoline-3-carboxylic acid ethyl ester 1071004-67-5 C15H19NO3Si 289.406 - 1
- 2
- 3
- 4
- 5
反应信息
-
作为反应物:描述:参考文献:名称:作为潜在的检查点激酶1(Chk1)抑制剂的2-芳基-2H-吡唑并[4,3-c]喹啉-3-酮的合成及其初步的构效关系研究。摘要:丝氨酸-苏氨酸检查点激酶1(Chk1)在细胞周期停滞中起着至关重要的作用,以响应DNA损伤。在过去的十年中,Chk1抑制剂已成为增强细胞毒性化学治疗剂抗肿瘤功效的新型治疗策略。在寻找新的Chk1抑制剂时,首次通过体外和计算机模拟方法评估了同类的2-芳基-2 H-吡唑并[4,3-c]喹啉-3-酮(PQ)。 。使用常规或微波加热以良好至极好的产率合成了总共30个PQ结构,突出显示其中14个是新的化学实体。值得注意的是,在这项初步研究中,两种化合物4e2和4h2已显示Chk1激酶的基础活性适度但显着降低。从这些初步结果开始,DOI:10.1080/14756366.2017.1404592
-
作为产物:描述:2-硝基苯甲酰乙酸乙酯 在 氢气 作用下, 以 四氢呋喃 、 甲苯 为溶剂, 20.0~100.0 ℃ 、101.33 kPa 条件下, 反应 12.0h, 生成 1,4-二氢-4-氧代-3-喹啉羧酸乙酯参考文献:名称:依伐卡托的高效合成摘要:依伐卡托的新型实用合成路线以克为单位描述。通过在芳族环上亲电加成两个叔丁基,可制备纯度为98.1%的3个步骤,收率61%的5-氨基-2,4-二叔丁基苯酚(高效液相色谱法)。3-二(2-氨基苯基)-3-氧代丙酸乙酯的分子内环化与二甲基甲酰胺的动力学力学分析用于在四个步骤中以54%的收率制备4-氧代-1,4-二氢喹啉-3-羧酸。依伐卡托是通过两个部分的缩合获得的,产率为71%,纯度为99.1%(高效液相色谱法)。DOI:10.1002/jhet.2931
-
作为试剂:描述:5-(Benzyloxy)-2,4-di-tert-butylaniline 、 1,4-二氢-4-氧代-3-喹啉羧酸乙酯 在 1,4-二氢-4-氧代-3-喹啉羧酸乙酯 作用下, 以71.3的产率得到N-(5-(Benzyloxy)-2,4-di-tert-butylphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide参考文献:名称:化合物的制备方法摘要:本发明提供了制备式I所示化合物的方法,该方法包括:(1)将式1所示化合物进行硝化反应,以便获得式2所示化合物;(2)利用苄基氯对式2所示化合物进行羟基保护处理,以便获得式3所示化合物;(3)将式3所示化合物进行还原反应,以便获得式4所示化合物;(4)将式5所示化合物与苯胺接触,以便获得式6所示化合物;(5)将式4所示化合物与式6所示化合物接触,以便获得式7所示化合物;(6)将式7所示化合物进行羟基脱保护反应,以便获得式I所示化合物。本发明的合成方法路线短、操作简单、原料易得、收率高、最终产物纯度高、适合工业化生产。公开号:CN103787968A
文献信息
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
COMPOSITIONS FOR TREATMENT OF CYSTIC FIBROSIS AND OTHER CHRONIC DISEASES申请人:Van Goor Fredrick F.公开号:US20110098311A1公开(公告)日:2011-04-28The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
[EN] HETEROCYCLIC DERIVATIVES FOR TREATMENT OF HYPERLIPIDEMIA AND RELATED DISEASES<br/>[FR] DERIVES HETEROCYCLIQUES POUR LE TRAITEMENT DE L'HYPERLIPIDEMIE ET DE MALADIES ASSOCIEES申请人:AVANIR PHARMACEUTICALS公开号:WO2005123686A1公开(公告)日:2005-12-29The present invention provides compositions adapted to enhance reverse cholesterol transport in mammals. The compositions are suitable for oral delivery and useful in the treatment and/or prevention of hypercholesterolemia, atherosclerosis and associated cardiovascular diseases.
-
一种哌尼诺安类似物及其制备方法和用途申请人:中国海洋大学公开号:CN108690024A公开(公告)日:2018-10-23本发明涉及一类哌尼诺安类似物,或其互变异构体、立体异构体、外消旋体、对映异构体的非等量混合物、几何异构体、溶剂化物、药学上可接受的盐或前药,以及包含该化合物的药物组合物。本发明还公开了这类化合物及其药物组合物作为药物,尤其是作为抗病毒、抗菌及抗寄生虫药物的用途。
-
[EN] AGENTS FOR USE IN THE TREATMENT OF CARDIOVASCULAR AND INFLAMMATORY DISEASES STRUCTURALLY BASED ON 4(1 H)-QUINOLONE<br/>[FR] AGENTS DESTINÉS À ÊTRE UTILISÉS DANS LE TRAITEMENT DE MALADIES CARDIOVASCULAIRES ET INFLAMMATOIRES AYANT UNE STRUCTURE BASÉE SUR LA 4(1H)-QUINOLONE申请人:UCL BUSINESS PLC公开号:WO2015189560A1公开(公告)日:2015-12-17The present invention provides a compound of formula I, a tautomer thereof, or a pharmaceutically acceptable salt or N-oxide thereof for use in the treatment or prevention of a cardiovascular disease or of an inflammatory disease or condition:本发明提供了一种式I的化合物,其互变异构体,或其药用可接受的盐或N-氧化物,用于治疗或预防心血管疾病或炎症性疾病或症状。
表征谱图
-
氢谱1HNMR
-
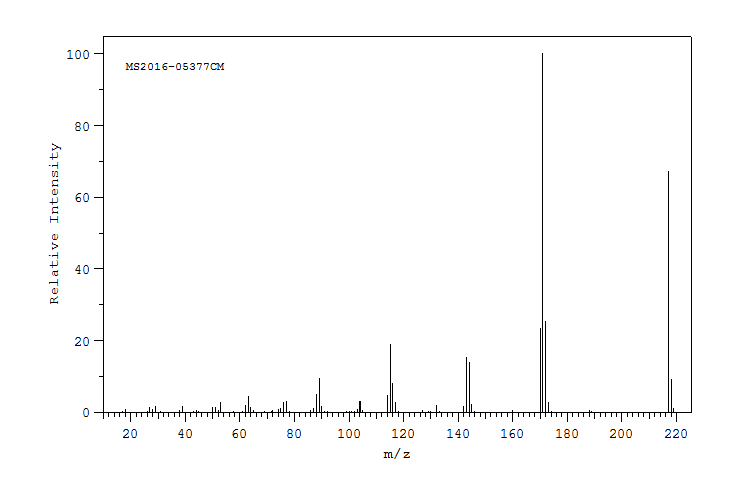
质谱MS
-
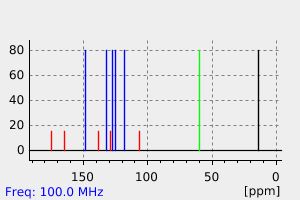
碳谱13CNMR
-
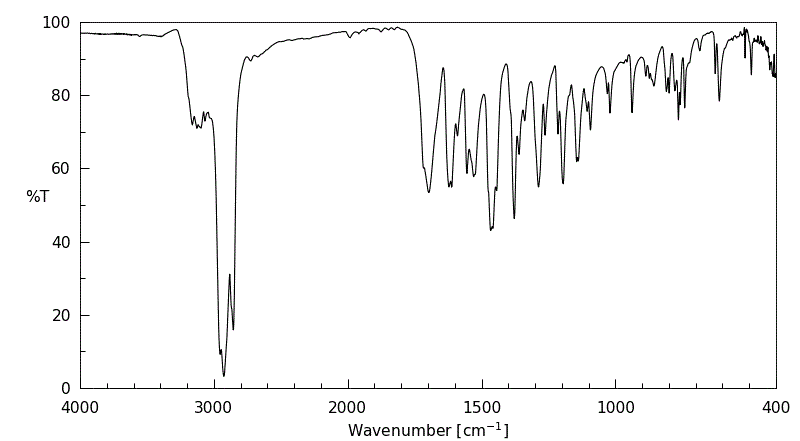
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43