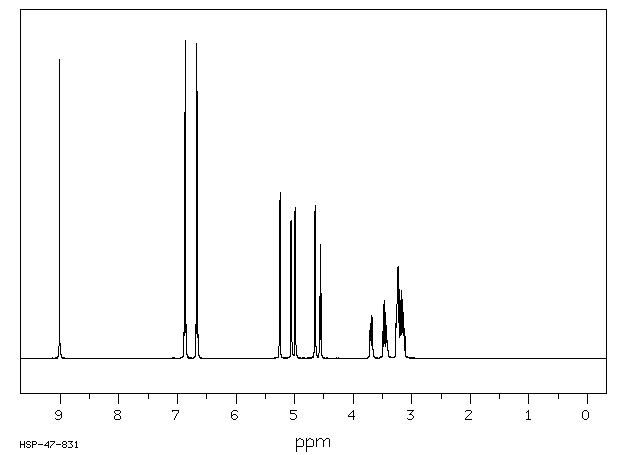
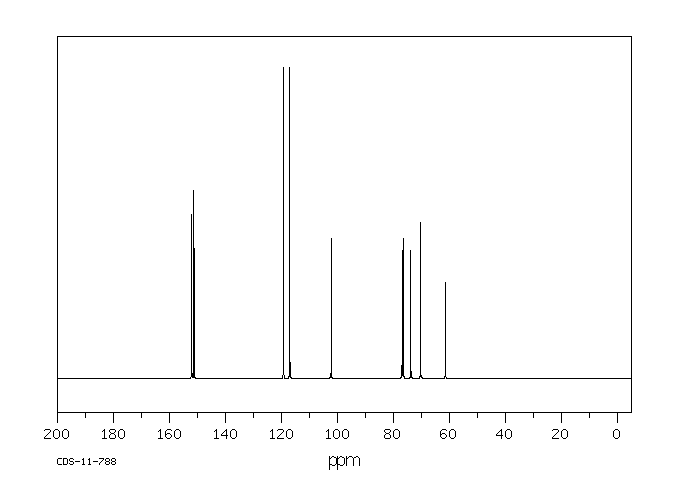
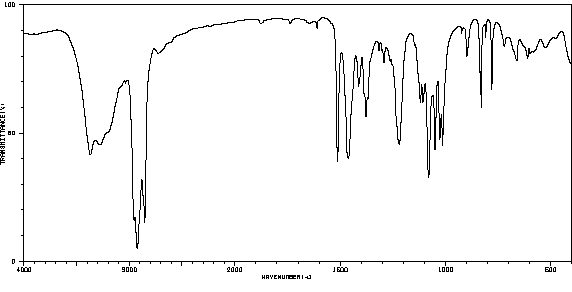
代谢
熊果苷在稀酸中容易
水解,生成
D-葡萄糖和
对苯二酚。预计口服给药的
熊果苷会通过胃酸轻易
水解为自由的
对苯二酚分子。
对苯二酚进一步代谢成为主要代谢物,
对苯二酚葡萄糖苷酸和
对苯二酚硫酸盐。
来源:DrugBank
毒理性
◉ 母乳喂养期间使用概述:少量矿物油可以出现在母乳中,这可能是由于长期吸收化妆品中的碳氢化合物。由于矿物油口服吸收不良,因此很少或没有矿物油会进入婴儿的血液或对母乳喂养的婴儿产生任何不良影响。一项小型研究支持母体矿物油对母乳喂养婴儿肠道的无影响。母乳喂养的母亲口服矿物油是可以接受的,尽管应避免反复使用,因为它可能导致脂溶性
维生素的缺乏。在或靠近乳房上使用矿物油或含有矿物油的软膏可能会使婴儿通过舔舐接触到高
水平的矿物石蜡。只有
水溶性的乳霜产品应该涂抹在乳房上。
◉ 对母乳喂养婴儿的影响:50位产后第一天的母亲接受了15毫升的矿物油或Magnolax(相当于3.75毫升的矿物油和900毫克的
氢氧化镁),尽管没有说明具体有多少人接受了每种产品。如果需要,随后的几天可以给予额外的剂量。他们母乳喂养的婴儿中没有出现明显异常的大便,尽管所有婴儿也接受了补充喂养。
◉ 对泌乳和母乳的影响:截至修订日期,没有找到相关的已发布信息。
来源:Drugs and Lactation Database (LactMed)
毒理性
熊果苷是
氢醌的β-
D-葡萄糖苷,是一种美白化妆品成分。与
熊果苷相比,
氢醌是一种更有效的美白剂,但显示出细胞毒性、肾毒性和遗传毒性。为了评估皮肤微
生物群是否能够将
熊果苷水解为
氢醌,我们测量了主要皮肤微
生物群:表皮葡萄球菌和
金黄色葡萄球菌的
水解活性。所有菌株都能
水解
熊果苷,活性为0.16-4.51 nmol/min/mg。
水解后的
氢醌比
熊果苷具有更强的1,1
-二苯基-2-苦基
肼自由基清除活性和
酪氨酸酶抑制作用。这些发现表明,正常皮肤微
生物群可能由于
氢醌的抗氧化作用而增加
熊果苷的美白效果。
来源:Hazardous Substances Data Bank (HSDB)
毒理性
这项研究展示了证据,表明
芦荟素和
熊果苷联合治疗可以通过不同的作用机制以协同方式抑制
酪氨酸酶活性。
芦荟素或
熊果苷以相似的方式抑制了人和蘑菇
酪氨酸酶的酶活性,其半抑制浓度(IC50)分别为0.1 mM和0.04 mM。酶动力学数据的林韦弗-伯克图显示,
芦荟素以非竞争性方式抑制
酪氨酸酶活性,其Ki值为5.3 mM,而
熊果苷则以竞争性方式抑制(Maeda, 1996)。然后我们研究了这些试剂联合治疗是否以协同方式抑制
酪氨酸酶活性。结果显示,在0.03 mM
熊果苷存在的情况下,0.01 mM
芦荟素使蘑菇
酪氨酸酶活性降低了对照组的80%,反之亦然。根据Burgi方法计算,抑制效果是协同的。综上所述,我们建议
芦荟素与
熊果苷联合可以通过非竞争性和竞争性抑制
酪氨酸酶活性的组合机制协同抑制
黑色素产生。
来源:Hazardous Substances Data Bank (HSDB)
毒理性
/SRP:/ 立即急救:确保已经进行了充分的中毒物清除。如果患者停止呼吸,开始人工呼吸,最好使用需求阀复苏器、袋阀面罩装置或口袋面罩,按训练操作。根据需要执行心肺复苏。立即用缓慢流动的
水冲洗受污染的眼睛。不要催吐。如果发生呕吐,让患者前倾或置于左侧(如果可能的话,头部向下)以保持呼吸道畅通,防止吸入。保持患者安静,维持正常体温。寻求医疗救助。 /
苯胺及其相关化合物/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
/SRP:/ 基本治疗:建立专利气道(如有需要,使用口咽或鼻咽气道)。如有必要,进行吸痰。密切观察呼吸不足的迹象,如有必要,进行辅助通气。通过非重复呼吸面罩以10至15升/分钟的速度给予
氧气。监测休克迹象,并在必要时进行治疗……预见癫痫发作并在必要时进行治疗……对于眼睛污染,立即用
水冲洗眼睛。在运输过程中,用0.9%的生理盐
水(NS)连续冲洗每只眼睛……不要使用催吐剂。对于误食,如果患者能够吞咽、有强烈的呕吐反射且不流口
水,则用
水冲洗口腔,并给予5毫升/千克,最多200毫升的
水进行稀释。给予
活性炭……/
苯胺及其相关化合物/
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
熊果苷被发现在胃肠道中被广泛吸收,在那里它主要被转化为
氢醌。
来源:DrugBank
吸收、分配和排泄
在健康志愿者单次服用210毫克
熊果苷后的前4小时内,尿液中回收了224.5微摩尔/升的
氢醌葡萄糖醛酸苷和182微摩尔/升的
氢醌硫酸盐。
来源:DrugBank
吸收、分配和排泄
无药物动力学数据可用。
来源:DrugBank
吸收、分配和排泄
无药代动力学数据可用。
来源:DrugBank
吸收、分配和排泄
熊果叶干
提取物(BLDE)单次口服剂量后,对16名健康志愿者进行了
熊果苷代谢物尿排泄的随机交叉设计研究。应用分为两组,一组使用薄膜包衣片(FCT),另一组使用
水溶液(AS)。尿液样本分析采用验证的高效
液相色谱方法(对羟基
苯酚)和验证的毛细管电泳方法(对羟基
苯酚-
葡萄糖醛酸苷、对羟基
苯酚-
硫酸盐)。两组从BLDE排入尿液中的总对羟基
苯酚当量相似。使用FCT时,64.8%的
熊果苷剂量被排泄;使用AS时,66.7%被排泄(p = 0.61)。对羟基
苯酚当量的最大平均尿浓度在AS组比FCT组略高,并且峰值出现得更早,尽管这没有达到统计学意义(Cmax = 1.6893 umol/mL 对比 1.1250 umol/mL,p = 0.13;tmax(t中点)= 3.60小时 对比 4.40小时,p = 0.38)。与AS相比,FCT的总对羟基
苯酚当量的相对
生物利用度为103.3%。受试者间的变异性很大。两组在检测到的代谢物模式(对羟基
苯酚、对羟基
苯酚-
葡萄糖醛酸苷和对羟基
苯酚-
硫酸盐)之间没有显著差异。
来源:Hazardous Substances Data Bank (HSDB)