4-氯二苯甲酮 | 134-85-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:74-76 °C (lit.)
-
沸点:195-196 °C/17 mmHg (lit.)
-
密度:1.1459 (rough estimate)
-
闪点:143°C
-
溶解度:可溶于氯仿(少许)、乙酸乙酯(少许)、甲醇(少许)
-
LogP:3.748 at 25℃
-
颜色/状态:Needles from alcohol
-
蒸汽压力:1.1X10-4 mm Hg at 25 °C (est)
-
保留指数:1850;1850;1850;1850
-
稳定性/保质期:
白色结晶粉末,熔点为75℃。
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:15
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
ADMET
安全信息
-
TSCA:Yes
-
危险品标志:Xn,Xi,C
-
安全说明:S22,S24/25,S26,S2637/39,S36/37/39,S45
-
危险类别码:R34
-
WGK Germany:2
-
海关编码:2914700090
-
危险品运输编号:UN 3261 8/PG 2
-
RTECS号:AM5978800
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:室温
SDS
: 4-Chlorobenzophenone
化学品俗名或商品名
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C13H9ClO
分子式
: 216.66 g/mol
分子量
成分 浓度
4-Chlorobenzophenone
-
化学文摘编号(CAS No.) 134-85-0
EC-编号 205-160-7
模块 4. 急救措施
4.1 必要的急救措施描述
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
在皮肤接触的情况下
用肥皂和大量的水冲洗。
在眼睛接触的情况下
用水冲洗眼睛作为预防措施。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 最重要的症状和影响,急性的和滞后的
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氯化氢气体
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止粉尘的生成。 防止吸入蒸汽、气雾或气体。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
扫掉和铲掉。 存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
人身保护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所来选择人体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 结晶
颜色: 棕灰色
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/熔点范围: 74 - 76 °C - lit.
f) 起始沸点和沸程
195 - 196 °C 在 23 hPa - lit.
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
4-氯二苯甲酮是一种无色到淡黄色的固体粉末,闪点为143℃,沸点为332℃。它能够溶于乙醚和热乙醇。
应用4-氯二苯甲酮通常以米白色或灰白色的晶体形式存在,是合成降血脂药物非诺贝特等医药和农药产品以及制备耐热性聚合物的重要原料,应用非常广泛。此外,作为一种重要的化工中间体,它还被广泛应用于医药、农药、染料以及其他有机合成中。
合成方法4-氯二苯甲酮可以通过以下步骤来合成:
- 取适量的4-氯苯甲酰氯(1.5 mmol)、苯基硼酸(1.25 mmol)和碳酸钠(133 mg,1.25 mmol),用氩气冲洗反应管,并密封好。
- 向试管中加入3 mL甲苯和3 mL水。
- 将反应容器置于保持在50℃的油浴中并搅拌混合物1小时。
- 反应完成后,将混合物转移到分液漏斗中,用20 mL乙醚稀释,并分离出水相。
- 依次用3 M HCl、5% KOH和盐水洗涤有机相,并使用无水硫酸镁干燥。
- 使用色谱级硅胶在真空下蒸发有机相,预吸附粗产物。
- 对产物进行柱色谱纯化(己烷-乙酸乙酯比例为30∶1、10∶1或5∶1),并最终得到标题化合物4-氯二苯甲酮。
4-氯二苯甲酮是一种类白色的结晶粉末。
用途这种物质常用于UV固化型涂料和油墨中,同时也用作医药、农药中间体。
生产方法上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4,4'-二氯二苯甲酮 4,4'-Dichlorobenzophenone 90-98-2 C13H8Cl2O 251.112 4-氨基-4-氯苯甲酮 4-amino-4'-chlorobenzophenone 4913-77-3 C13H10ClNO 231.681 4-氯二苯基甲烷 4-chlorophenyl(phenyl)methane 831-81-2 C13H11Cl 202.683 4-氨基二苯甲酮 (4-aminophenyl)(phenyl)methanone 1137-41-3 C13H11NO 197.236 4-碘二苯酮 4-iodobenzophenone 6136-66-9 C13H9IO 308.118 4-氯苯甲醛 4-chlorobenzaldehyde 104-88-1 C7H5ClO 140.569 4-苯甲酰基苯甲酰氯 4-benzoylbenzoyl chloride 39148-58-8 C14H9ClO2 244.677 —— 4-chlorothiobenzhydrol 93551-92-9 C13H11ClS 234.749 4-氯二苯氯甲烷 1-chloro-4-(chloro(phenyl)methyl)benzene 134-83-8 C13H10Cl2 237.128 1-氯-4-(1-苯基乙基)苯 1-chloro-4-(1-phenylethyl)benzene 60617-89-2 C14H13Cl 216.71 1-(1-(4-氯苯基)乙烯基)苯 1-(4-chlorophenyl)-1-phenylethene 18218-20-7 C14H11Cl 214.694 4-氯二苯甲醇 (4-chlorophenyl)phenylmethanol 119-56-2 C13H11ClO 218.683 —— p-chlorobenzophenone imine 41839-60-5 C13H10ClN 215.682 —— 4-chloro-thiobenzophenone 2484-99-3 C13H9ClS 232.733 4-氯-Alpha-苯基-苯甲胺 p-chlorobenzhydrylamine 28022-43-7 C13H12ClN 217.698 (S)-(4-氯苯基)苯基甲胺 (S)-(+)-4-chlorophenyl(phenyl)methylamine 163837-32-9 C13H12ClN 217.698 (-)-4-氯-Alpha-苯基-苯甲胺 (R)-(4-chlorophenyl)(phenyl)methylamine 163837-57-8 C13H12ClN 217.698 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-氯-4'-羟基二苯甲酮 4-chloro-4'-hydroxybenzophenone 42019-78-3 C13H9ClO2 232.666 二苯甲酮 benzophenone 119-61-9 C13H10O 182.222 4-甲基二苯甲酮 4-Methylbenzophenone 134-84-9 C14H12O 196.249 4-氯二苯基甲烷 4-chlorophenyl(phenyl)methane 831-81-2 C13H11Cl 202.683 4-苯甲酰基苯甲醛 4-benzoylbenzaldehyde 20912-50-9 C14H10O2 210.232 (4-苄基苯基)(苯基)甲酮 4-benzylbenzophenone 58280-04-9 C20H16O 272.346 4-氨基二苯甲酮 (4-aminophenyl)(phenyl)methanone 1137-41-3 C13H11NO 197.236 胆碱非诺贝特杂质1 4'-chloro-2-hydroxybenzophenone 2985-79-7 C13H9ClO2 232.666 4-碘二苯酮 4-iodobenzophenone 6136-66-9 C13H9IO 308.118 —— (4-mercaptophenyl)(phenyl)methanone 1620-94-6 C13H10OS 214.288 4-羟基-二苯甲酮 4-Hydroxybenzophenone 1137-42-4 C13H10O2 198.221 —— [18F]-4-fluorobenzophenone 115216-92-7 C13H9FO 199.214 4-乙基苯甲酮 4-ethylbenzophenone 18220-90-1 C15H14O 210.276 [4-(羟甲基)苯基]-苯基甲酮 4-(hydroxymethyl)benzophenone 81449-01-6 C14H12O2 212.248 —— 4-vinylbenzophenone 3139-85-3 C15H12O 208.26 4-氰基苯甲酮 p-cyanobenzophenone 1503-49-7 C14H9NO 207.232 —— 4-benzhydrylbenzophenone 7375-38-4 C26H20O 348.444 —— 3,4'-diamino-4-chloro-benzophenone 71969-52-3 C13H11ClN2O 246.696 —— ((4-chlorophenyl)methylene)dibenzene 69361-54-2 C19H15Cl 278.781 —— 4-chloro-2-hydroxybenzophenone 2985-80-0 C13H9ClO2 232.666 2-(4-苯甲酰基苯基)乙腈 2-(4-benzoylphenyl)acetonitrile 21192-61-0 C15H11NO 221.258 —— (4'-chloro-[1,1'-biphenyl]-4-yl)(phenyl)methanone 63242-15-9 C19H13ClO 292.765 4-异丙基二苯甲酮 4-isopropylbenzophenone 18864-76-1 C16H16O 224.302 —— (4-(difluoromethyl)phenyl)(phenyl)methanone 64747-73-5 C14H10F2O 232.23 [4-(甲基氨基)苯基]-苯基甲酮 (4-(methylamino)phenyl)(phenyl)methanone 26178-74-5 C14H13NO 211.263 —— (4-chloro-2-hydroxyphenyl)(phenyl)methanone 1428244-95-4 C13H9ClO3 248.666 1-(4-苯甲酰基苯基)乙酮 4-acetylbenzophenone 53689-84-2 C15H12O2 224.259 4-甲氧基二苯甲酮 4-Methoxybenzophenone 611-94-9 C14H12O2 212.248 (4-丁基苯基)(苯基)甲酮 4-n-butylbenzophenone 55363-57-0 C17H18O 238.329 —— 4-isobutylbenzophenone 64357-65-9 C17H18O 238.329 4-氯二苯氯甲烷 1-chloro-4-(chloro(phenyl)methyl)benzene 134-83-8 C13H10Cl2 237.128 1-(溴苯甲基)-4-氯苯 4-chlorobenzhydryl bromide 948-54-9 C13H10BrCl 281.579 1-(1-(4-氯苯基)乙烯基)苯 1-(4-chlorophenyl)-1-phenylethene 18218-20-7 C14H11Cl 214.694 (4’-甲基联苯-4-基)(苯基)甲酮 4-benzoyl-4'-methylbiphenyl 63283-56-7 C20H16O 272.346 4-苯基二苯甲酮 biphenyl-4-yl-phenyl-methanone 2128-93-0 C19H14O 258.32 —— 4,4'-dibenzoyl-1,1'-biphenyl 33090-29-8 C26H18O2 362.428 对二甲氨基二苯甲酮 4-(Dimethylamino)benzophenone 530-44-9 C15H15NO 225.29 4-氯二苯甲醇 (4-chlorophenyl)phenylmethanol 119-56-2 C13H11ClO 218.683 (alphaS)-4-氯-alpha-苯基苯甲醇 (S)-4-chlorobenzhydrol 101402-04-4 C13H11ClO 218.683 (R)-4-氯二苯基甲醇 (R)-(4-chlorophenyl)phenylmethanol 123535-85-3 C13H11ClO 218.683 —— 4-benzoyldiphenylamine 4058-17-7 C19H15NO 273.334 —— phenyl(4-(p-tolylamino)phenyl)methanone 42872-23-1 C20H17NO 287.361 —— (4-neopentylphenyl)(phenyl)methanone 64278-15-5 C18H20O 252.356 4-苯甲酰基-4'-甲基-二苯硫醚 phenyl(4-(p-tolylthio)phenyl)methanone 83846-85-9 C20H16OS 304.412 —— phenyl(4-(phenylthio)phenyl)methanone 6317-78-8 C19H14OS 290.386 —— trans-4-Benzoylstilbene 20488-44-2 C21H16O 284.357 苯基-[4-(2-苯基乙炔基)苯基]甲酮 4-(phenylethynyl)benzophenone 57542-59-3 C21H14O 282.342 —— 4-ethylaminobenzophenone —— C15H15NO 225.29 4-苯氧基苯甲酮 p-phenoxybenzophenone 6317-73-3 C19H14O2 274.319 —— bis-(4-benzoylphenyl) ether 6966-89-8 C26H18O3 378.427 —— 4-chloro-thiobenzophenone 2484-99-3 C13H9ClS 232.733 4-苯甲酰苯硼酸 (4-benzoylphenyl)boronic acid 268218-94-6 C13H11BO3 226.04 (-)-4-氯-Alpha-苯基-苯甲胺 (R)-(4-chlorophenyl)(phenyl)methylamine 163837-57-8 C13H12ClN 217.698 (S)-(4-氯苯基)苯基甲胺 (S)-(+)-4-chlorophenyl(phenyl)methylamine 163837-32-9 C13H12ClN 217.698 4-氯-Alpha-苯基-苯甲胺 p-chlorobenzhydrylamine 28022-43-7 C13H12ClN 217.698 - 1
- 2
- 3
- 4
- 5
- 6
反应信息
-
作为反应物:描述:4-氯二苯甲酮 在 氯化亚砜 、 水 、 sodium methylate 、 甲基三辛基氯化铵 、 sodium hydroxide 作用下, 以 甲苯 为溶剂, 反应 14.0h, 生成 cetirizine dihydrochloride参考文献:名称:西替利嗪的新生产工艺摘要:通过新的中间体2-(2- {2- {4-[(4-氯苯基)(苯基)甲基]哌嗪-1-基}乙氧基)-N,N-二甲基乙酰胺二盐酸盐的合成新工艺制备西替利嗪的新方法阐述了2- {4-[(4-氯苯基)(苯基)甲基]哌嗪-1-基}乙醇与2-氯-N,N-二甲基乙酰胺的O-烷基化。所得酰胺的水解和随后的成盐作用提供了西替利嗪二盐酸盐。DOI:10.1021/op300009y
-
作为产物:描述:参考文献:名称:AIBN在存在双氧的情况下引发了苄基sp 3 C H和C C键的官能化摘要:通过AIBN / O 2催化剂体系实现了sp 3 C H键的官能化和C C键的裂解,在温和的反应条件下提供了一系列的二苯甲酮。机理研究表明,过氧化物中间体参与了这一转变,在二苯基甲烷的情况下,sp 3 C C键通过过氧化物重排而断裂,这可能提供了一种断裂相对较强的C C键并被应用的新方法。更一般的C C键活化。DOI:10.1016/j.tetlet.2020.152806
-
作为试剂:参考文献:名称:通过 EDA 催化的 ET-HAT 过程产生可见光驱动的甲自由基摘要:通过在电子供体-受体(EDA)复合物中使用光激发电子转移(ET)来驱动甲氢化体(R 3 GeH)的氢原子转移(HAT),可以轻松产生甲锗自由基。使用催化量的市售硫醇和二苯甲酮衍生物,ET-HAT 循环在蓝光照射下顺利进行,无需任何光催化剂。DOI:10.1002/chem.202401546
文献信息
-
Efficient one-pot transformation of aminoarenes to haloarenes using halodimethylisulfonium halides generated in situ作者:Woonphil Baik、Wanqiang Luan、Hyun Joo Lee、Cheol Hun Yoon、Sangho Koo、Byeong Hyo KimDOI:10.1139/v05-026日期:2005.3.1
Halodimethylsulfonium halide 1, which is readily formed in situ from hydrohaloic acid and DMSO, is a good nucleophilic halide. This activated nucleophilic halide rapidly converts aryldiazonium salt prepared in situ by the same hydrohaloic acid and nitrite ion to aryl chlorides, bromides, or iodides in good yield. The combined action of nitrite ion and hydrohaloic acid in DMSO is required for the direct transformation of aromatic amines, which results in the production of aryl halides within 1 h. Substituted compounds with electron-donating or -withdrawing groups or sterically hindered aromatic amines are also smoothly transformed to the corresponding aromatic halides. The only observed by-product is the deaminated arene (usually <7%). The isolated aryldiazonium salts can also be converted to the corresponding aryl halides using 1. The present method offers a facile, one-step procedure for transforming aminoarenes to haloarenes and lacks the environmental pollutants that usually accompany the Sandmeyer reaction using copper halides. Key words: aminoarenes, haloarenes, halodimethylsulfonium halide, halogenation, amination.
卤二甲基亚砜卤化物1是一种良好的亲核卤化物,可在现场由氢卤酸和二甲亚砜形成。这种活化的亲核卤化物迅速将由相同的氢卤酸和亚硝酸根在现场制备的芳基重氮盐转化为芳基氯化物、溴化物或碘化物,收率较高。在DMSO中,亚硝酸根和氢卤酸的联合作用是直接转化芳香胺的必要条件,从而在1小时内产生芳基卤化物。带有电子给体或吸引基团或有立体位阻的芳香胺的取代化合物也可顺利转化为相应的芳香卤化物。观察到的唯一副产物是去氨基芳烃(通常<7%)。孤立的芳基重氮盐也可以使用1转化为相应的芳基卤化物。该方法提供了一种简便的、一步法的程序,用于将氨基芳烃转化为卤代芳烃,并且不伴随通常伴随使用铜卤化物进行桑迈尔反应的环境污染物。关键词:氨基芳烃,卤代芳烃,卤二甲基亚砜卤化物,卤化,胺化。 -
Engineering an Enantioselective Amine Oxidase for the Synthesis of Pharmaceutical Building Blocks and Alkaloid Natural Products作者:Diego Ghislieri、Anthony P. Green、Marta Pontini、Simon C. Willies、Ian Rowles、Annika Frank、Gideon Grogan、Nicholas J. TurnerDOI:10.1021/ja4051235日期:2013.7.24catalytic methods for the production of enantiomerically pure chiral amines is a key challenge facing the pharmaceutical and fine chemical industries. This challenge is highlighted by the estimate that 40-45% of drug candidates contain a chiral amine, fueling a demand for broadly applicable synthetic methods that deliver target structures in high yield and enantiomeric excess. Herein we describe the development开发用于生产对映异构纯手性胺的经济高效且可持续的催化方法是制药和精细化工行业面临的关键挑战。据估计,40-45% 的候选药物含有手性胺,这突显了这一挑战,这推动了对以高产率和对映体过量提供目标结构的广泛适用的合成方法的需求。在此,我们描述了来自黑曲霉 (MAO-N) 的单胺氧化酶变体“工具箱”的开发和应用,该变体显示出显着的底物范围和对空间要求基序的耐受性,包括一个新变体,它对含有以下物质的底物表现出高活性和对映选择性氨基二苯甲烷(二苯甲基胺)模板。通过将合理的结构导向工程与高通量筛选相结合,可以扩大 MAO-N 的底物范围以适应含有大量芳基取代基的胺底物。这些工程化的 MAO-N 生物催化剂已应用于去消旋反应,用于高效不对称合成仿制药活性药物成分索利那新和左西替利嗪以及天然产物 (R)-coniine、(R)-eleagnine 和 (R)-leptaflorine . 我们还报告了一种新的
-
Ionic liquid-mediated benzoyl transfer-coupling in the Suzuki and Sonogashira reactions and aryl transfer-coupling by decarbonylative Heck reaction, using N-Benzoyl-saccharin (NBSac) as reagent作者:Shruti S. Malunavar、Suraj M. Sutar、Pavankumar Prabhala、Rajesh G. Kalkhambkar、Kenneth K. LaaliDOI:10.1016/j.tetlet.2020.151987日期:2020.6of N-benzoyl-saccharin (NBSac) as reagent for selective benzoyl transfer-coupling in the Suzuki reaction in BMIM-IL/[PAIM][NTf2] as solvent/base, and in the Sonogashira reaction employing guanidinium-IL (GIL) as solvent, are demonstrated. Decarbonylative aryl transfer-coupling occurs in the Heck reaction employing GIL as solvent. The reactions are catalyzed by Pd(OAc)2 or NiCl2(dppp), are performed
-
Efficient Reductive Deoximation by Tungsten(VI) Chloride (WCl<sub>6</sub>) or Molybdenum(V) Chloride (MoCl<sub>5</sub>) in the Presence of Zn Powder in CH<sub>3</sub>CN作者:Habib Firouzabadi、Arezu Jamalian、Babak KarimiDOI:10.1246/bcsj.75.1761日期:2002.8Tungsten(VI) chloride (WCl6) or molybdenum(V) chloride (MoCl5) in the presence of zinc powder in CH3CN provides an efficient and facile procedure for the deprotection of oximes to their corresponding aldehydes and ketones in high yields.
-
A simple and efficient oxidation of alcohols with ruthenium on carbon作者:Shigeki Mori、Masato Takubo、Kazuya Makida、Takayoshi Yanase、Satoka Aoyagi、Tomohiro Maegawa、Yasunari Monguchi、Hironao SajikiDOI:10.1039/b908451g日期:——A simple, efficient, and environmentally-friendly heterogeneous Ru/C-catalyzed oxidation of secondary and primary alcohols without additives under an atmosphere of oxygen has been established.已经建立了在氧气气氛下没有添加剂的简单,有效和环境友好的Ru / C多相Ru / C催化仲醇和伯醇的氧化方法。
表征谱图
-
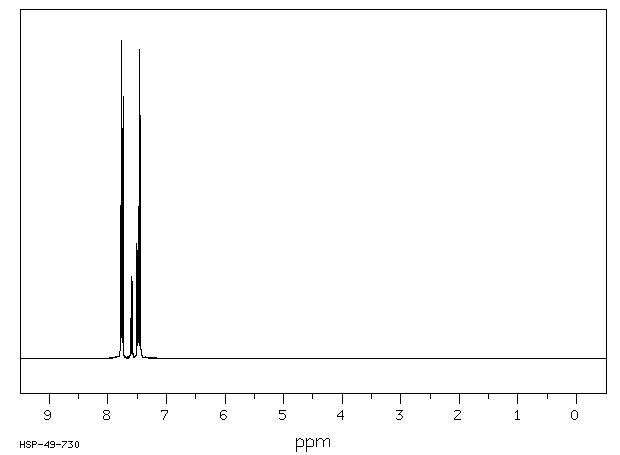
氢谱1HNMR
-
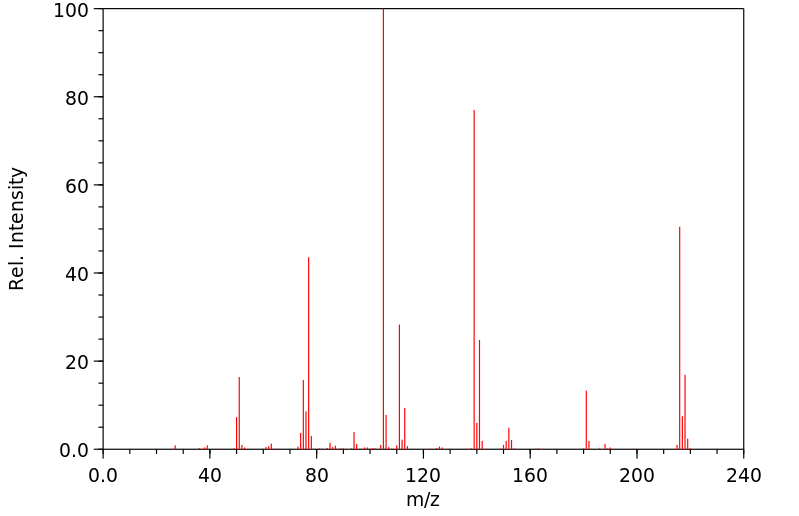
质谱MS
-
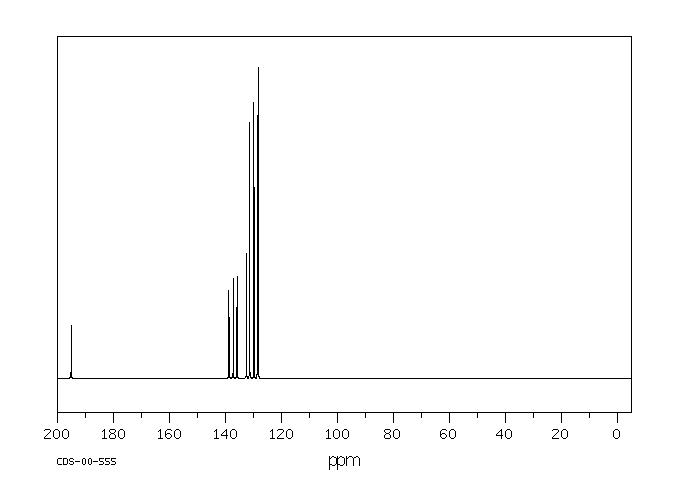
碳谱13CNMR
-
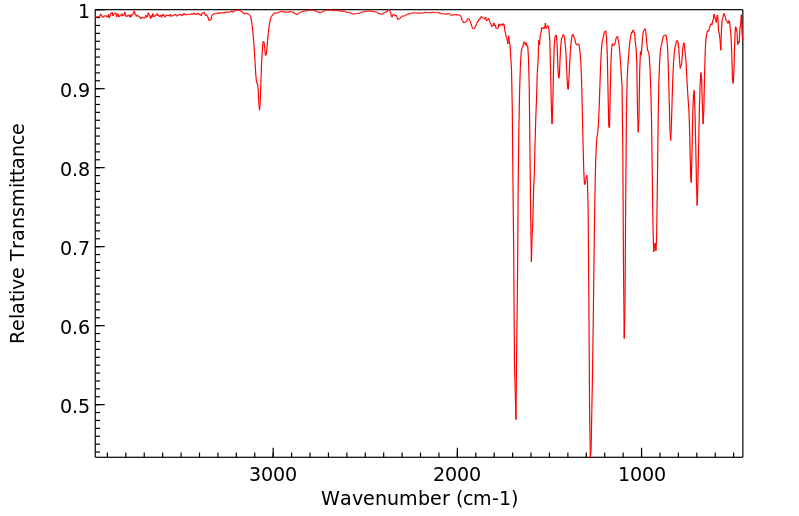
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息