2',3'-二脱氧肌苷 | 69655-05-6
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:193-195 °C
-
沸点:193-195 C
-
比旋光度:D25 -26.3° (c = 10 in water)
-
密度:1.2917 (rough estimate)
-
溶解度:溶于DMSO或甲醇
-
物理描述:2',3'-dideoxyinosine appears as fluffy white solid or powder. Condenses at 347°F and darkens at approximately 572°F. Odorless. (NTP, 1992)
-
颜色/状态:White solid
-
蒸汽压力:6.6 mm Hg @ 25 °C /Estimated/
-
亨利常数:Henry's Law constant = 2.4X10-20 atm-cu m/mol @ 25 °C /Estimated/
-
稳定性/保质期:
Didanosine unbuffered pediatric powder for oral solution should be stored at 15-30 °C. Following reconstitution with water and admixture with a liquid antacid as directed, didanosine pediatric oral suspensions contain 10 mg of the drug per ml and are stable for 30 days when refrigerated at 2-8 °C. Reconstituted and admixed pediatric oral suspensions of didanosine should be stored in tightly closed, flint glass bottles with child resistant closures and refrigerated at 2-8 °C. Unused portions of reconstituted and admixed pediatric didanosine oral suspension should be discarded after 30 days.
-
旋光度:(c = 1, H2O) [a]20D = -25.7 ± 2°
-
解离常数:pKa= 9.13
计算性质
-
辛醇/水分配系数(LogP):-1.2
-
重原子数:17
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:88.7
-
氢给体数:2
-
氢受体数:5
ADMET
安全信息
-
危险品标志:C
-
安全说明:S26,S27,S36/37/39,S45
-
危险类别码:R34
-
WGK Germany:2
-
海关编码:29335990,2942000000
-
RTECS号:NM7460700
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
SDS
制备方法与用途
去羟肌苷对齐多夫定耐药的HIV病毒株仍有作用。主要适用于不能耐受齐多夫定、在应用齐多夫定过程中产生耐药性或病情反复恶化的艾滋病患者。
药理作用去羟肌苷是一种HIV反转录酶抑制剂,在细胞酶的作用下转化为具有抗病毒活性的代谢物双去氧三磷酸腺苷而起作用,掺入病毒DNA,使病毒的延长终止。其作用机制与齐多夫定相似。不能预防HIV通过性接触或血液污染造成的传染。与其他抗病毒药物联合使用(鸡尾酒疗法)用于治疗I型HIV感染。
抗病毒药和抗艾滋病药地达诺辛是一种抗病毒药和抗艾滋病药,又称惠妥滋、地丹诺辛、二脱氧胸苷、双脱氧肌苷或去羟肌苷。为人免疫缺陷病毒(HIV)复制抑制剂,干扰和抑制病毒逆转录酶而阻止病毒的复制,作用机制与齐多夫定相似,但活性更高且选择性更强。由人工化学合成,在1991年11月首次在美国上市,为美国第二个被批准用来治疗HIV感染的药物,作为齐多夫定的替代品。是2′,3′-双脱氧腺苷(ddA)的前体药。
本品在病毒感染细胞内通过细胞酶的作用转化为有抗病毒活性的代谢物三磷酸双脱氧腺苷(ddA-TP)。ddA-TP是一种选择性很强的反转录酶抑制剂,干扰和抑制病毒的复制。临床使用后可使病情改善、CD4+ T淋巴细胞计数增加、HIV RNA水平降低等。
合成方法去羟肌苷可以通过多种合成路线制备:
- 第一种合成路径:将化合物进行脱保护基反应,经过多次纯化步骤最终得到产品。该过程包括脱保护、氢化和醇解等多个关键步骤。
- 第二种合成路径:通过有机溶剂中的酰氯化、取代及还原等工艺,实现从肌苷到去羟肌苷的转化。
- 第三种合成路径:采用特定的化学反应(5’-O-苯甲酰化、3’-0-(1-咪唑基)硫代羰基化)完成目标产物的合成。
- 第四种合成路径:通过微生物发酵技术,利用不同菌株进行生产。该方法包括对6-羟基嘌呤及其衍生物的发酵过程。
每一种方法都体现了去羟肌苷工业化生产的科学性和复杂性,适用于不同的需求和条件。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2'-脱氧肌苷 2-deoxyinosine 890-38-0 C10H12N4O4 252.23 肌苷 inosine 58-63-9 C10H12N4O5 268.229 (2S,5R)-5-(6-氯-9H-嘌呤-9-基)四氢-2-呋喃甲醇 β-9-(2,3-dideoxyribofuranosyl)-6-chloropurine 120503-34-6 C10H11ClN4O2 254.676 2',3'-双脱氧腺苷 2',3'-Dideoxyadenosine 4097-22-7 C10H13N5O2 235.246 —— [(S)-5-(6-Amino-purin-9-yl)-tetrahydro-furan-2-yl]-methanol —— C10H13N5O2 235.246 [(2S,5R)-5-(6-氧代-3,6-二氢-9H-嘌呤-9-基)四氢-2-呋喃基]甲基乙酸酯 5'-O-acetyl-2',3'-dideoxyinosine 130676-58-3 C12H14N4O4 278.268 2'-脱氧腺苷 2'-Deoxyadenosine 958-09-8 C10H13N5O3 251.245 —— 1-(2-deoxy-α,β-D-erythro-pentofuranosyl)imidazo[4,5-d]pyridazine-4(5H)-one —— C10H12N4O4 252.23 —— D-2',3'-dideoxy-5'-O-tert-butyldimethylsilylinosine 177779-56-5 C16H26N4O3Si 350.493 2'-beta-氟-2',3'-二脱氧腺苷 iodenosine 110143-10-7 C10H12FN5O2 253.236 —— 5'-benzoyl-2',3'-dideoxyinosine 117312-02-4 C17H16N4O4 340.338 —— 1-(5-O-tert-butyldimethylsilyl-2-deoxy-β-D-erythro-pentofuranosyl)imidazo[4,5-d]pyridazin-4(5H)-one 260562-59-2 C16H26N4O4Si 366.492 腺苷 adenosine 58-61-7 C10H13N5O4 267.244 —— 5'-O-[3-(trifluoromethyl)benzoyl]-2',3'-dideoxyinosine —— C18H15F3N4O4 408.337 —— N 1 ,5'-O-dibenzyl-2',3'-dideoxyinosine810694-79-2 C24H24N4O3 416.48 5’-O-[双(4-甲氧基苯基)苯基甲基]-2’-脱氧肌苷 5'-O-(4,4'-dimethoxytrityl)-2'-deoxyinosine 93778-57-5 C31H30N4O6 554.602 —— D-5'-O-tert-butyldimethylsilylinosine 119794-34-2 C16H26N4O5Si 382.492 —— 5'-(O-tert-butyldimethylsilyl)adenosine 69530-93-4 C16H27N5O4Si 381.507 2,3-二脱氧-2,3-二氢腺苷 2',3'-didehydro-2',3'-dideoxyadenosine 7057-48-9 C10H11N5O2 233.23 2’,3’-双脱氧双脱氢肌苷 2',3'-didehydro-2',3'-dideoxyinosine 42867-68-5 C10H10N4O3 234.214 —— 5'-O-(tert-butyldimethylsilyl)-2',3'-didehydro-2',3'-dideoxyadenosine 119794-33-1 C16H25N5O2Si 347.492 —— D-2',3'-didehydro-2',3'-dideoxy-5'-O-tert-butyldimethylsilylinosine 119818-51-8 C16H24N4O3Si 348.477 —— 2',3',5'-tri-O-[3-(trifluoromethyl)benzoyl]inosine —— C34H21F9N4O8 784.548 - 1
- 2
- 3
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 6-(3-methyl-2-butenyloxy)-9-(2',3'-dideoxy-β-D-ribofuranosyl)purine 1237496-82-0 C15H20N4O3 304.349 美他卡韦 2-amino-6-methoxy-9-(2,3-dideoxy-β-D-glyceropentofuranosyl)purine 120503-45-9 C11H15N5O3 265.272 —— 9-(2,3-dideoxy-β-D-ribofuranosyl)-6-methylpurine —— C11H14N4O2 234.258 (2S,5R)-5-(6-氯-9H-嘌呤-9-基)四氢-2-呋喃甲醇 β-9-(2,3-dideoxyribofuranosyl)-6-chloropurine 120503-34-6 C10H11ClN4O2 254.676 2',3'-双脱氧腺苷 2',3'-Dideoxyadenosine 4097-22-7 C10H13N5O2 235.246 [(2S,5R)-5-(6-氧代-3,6-二氢-9H-嘌呤-9-基)四氢-2-呋喃基]甲基乙酸酯 5'-O-acetyl-2',3'-dideoxyinosine 130676-58-3 C12H14N4O4 278.268 —— 5'-succinoyl-2',3'-dideoxyinosine 142894-17-5 C14H16N4O6 336.304 2,6-二氨基-2',3'-二脱氧嘌呤-9-呋喃核糖苷 2,6-diamino-9-(2,3-dideoxy-β-D-glycero-pentofuranosyl)purine 107550-73-2 C10H14N6O2 250.26 —— 2',3'-dideoxy-5'-hydrogen hexadecanedioate inosine 879015-25-5 C26H40N4O6 504.627 —— 5'-octanoyl-2',3'-dideoxyinosine 142894-15-3 C18H26N4O4 362.429 —— 2',3'-dideoxyinosine-5'-yl-O-ethyl carbonate —— C13H16N4O5 308.294 —— 2',3'-dideoxyinosine-5'-yl-O-butyl carbonate 1612888-54-6 C15H20N4O5 336.348 —— 2',3'-dideoxyinosine-5'-yl-O-pentyl carbonate 1612888-55-7 C16H22N4O5 350.374 —— 2',3'-dideoxyinosine-5'-yl-O-hexyl carbonate 1612888-56-8 C17H24N4O5 364.401 —— 5'-Boc-2',3'-dideoxyinosine 879015-29-9 C15H20N4O5 336.348 2',3'-二脱氧鸟苷 2',3'-dideoxyguanosine 85326-06-3 C10H13N5O3 251.245 [(2S,5R)-5-(6-氨基-2-氟嘌呤-9-基)四氢呋喃-2-基]甲醇 2-fluoro-9-(2,3-dideoxy-β-D-glycero-pentofuranosyl)adenine 114849-59-1 C10H12FN5O2 253.236 —— 1-O-[[(2S,5R)-5-(6-oxo-1H-purin-9-yl)oxolan-2-yl]methyl] 6-O-(2,2,2-trichloroethyl) hexanedioate 1188435-94-0 C18H21Cl3N4O6 495.747 —— 1-O-[[(2S,5R)-5-(6-oxo-1H-purin-9-yl)oxolan-2-yl]methyl] 9-O-(2,2,2-trichloroethyl) nonanedioate 1188435-95-1 C21H27Cl3N4O6 537.828 —— 1-O-[[(2S,5R)-5-(6-oxo-1H-purin-9-yl)oxolan-2-yl]methyl] 12-O-(2,2,2-trichloroethyl) dodecanedioate 1188435-96-2 C24H33Cl3N4O6 579.908 3'-脱氧肌苷 3'-deoxyinosine 13146-72-0 C10H12N4O4 252.23 —— D-2',3'-dideoxy-5'-O-tert-butyldimethylsilylinosine 177779-56-5 C16H26N4O3Si 350.493 —— (2S,5R)-5-(6-oxo-1H-purin-9-yl)oxolane-2-carbaldehyde 1401239-39-1 C10H10N4O3 234.214 —— 5'-benzoyl-2',3'-dideoxyinosine 117312-02-4 C17H16N4O4 340.338 —— 4-nitrophenyl (((2S,5R)-5-(6-oxo-3H-purin-9(6H)-yl)tetrahydrofuran-2-yl)methyl) carbonate —— C17H15N5O7 401.335 —— 2',3'-Dideoxy-8-hydroxyinosine 131265-48-0 C10H12N4O4 252.23 —— 5'-O[4-(Diethoxyphosphoryl)-4,4-difluorobutyl]-2',3'-dideoxyinosine —— C18H27F2N4O6P 464.406 —— methyl [methoxy-[[(2S,5R)-5-(6-oxo-1H-purin-9-yl)tetrahydrofuran-2-yl]methoxy]phosphinothioyl]formate —— C13H17N4O6PS 388.341 —— 11-{4-[(2S,5R)-5-(6-Oxo-1,6-dihydro-purin-9-yl)-tetrahydro-furan-2-ylmethoxycarbonyl]-butyl}-1,4,8,11-tetraaza-cyclotetradecane-1,4,8-tricarboxylic acid tri-tert-butyl ester —— C40H66N8O10 819.012 —— 11-{4-[(2S,5R)-5-(6-Oxo-1,6-dihydro-purin-9-yl)-tetrahydro-furan-2-ylmethoxycarbonyl]-butyryl}-1,4,8,11-tetraaza-cyclotetradecane-1,4,8-tricarboxylic acid tri-tert-butyl ester —— C40H64N8O11 832.995 —— 2',3'-dideoxyinosylyl-(5'->5')-N4-palmitoyl-2',3'-dideoxycytidine —— C35H54N7O9P 747.829 —— 5-O-[[(2R,5S)-5-(4-acetamido-2-oxopyrimidin-1-yl)-1,3-oxathiolan-2-yl]methyl] 1-O-[[(2S,5R)-5-(6-oxo-1H-purin-9-yl)oxolan-2-yl]methyl] pentanedioate 1058191-55-1 C25H29N7O9S 603.613 —— (Rp)- and (Sp)-4-butanoyloxybenzyl (3'-azido-3'-deoxythymidin-5-yl) (2',3'-dideoxyinosin-5'-yl) phosphate —— C31H36N9O11P 741.654 —— (Rp)- and (Sp)-4-pivaloyloxybenzyl (3'-azido-3'-deoxythymidin-5-yl) (2',3'-dideoxyinosin-5'-yl) phosphate —— C32H38N9O11P 755.681 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:描述:参考文献:名称:使用氢同位素交换有效地获得氘代和Tri化的Nucleobase药品和寡核苷酸。摘要:描述了一种有效的氢-同位素交换核碱基衍生物的通用方法。在温和的反应条件下,以钌纳米颗粒为催化剂,并以D 2或T 2为同位素源,该反应具有较宽的底物范围和较高的耐溶剂性。这种新颖的方法有助于在药物发现和开发中使用基本的诊断工具:具有高比活性的tri代药物和适合在LC-MS定量过程中用作内部标准品的氘代寡核苷酸。DOI:10.1002/anie.201813946
-
作为产物:描述:Methanesulfonic acid (2R,3R,4R,5R)-5-hydroxymethyl-4-methanesulfonyloxy-2-(6-oxo-1,6-dihydro-purin-9-yl)-tetrahydro-furan-3-yl ester 在 钯 Dowex anion-exchange resin 、 氢气 、 potassium hydrogencarbonate 作用下, 以 水 、 异丙醇 为溶剂, 70.0 ℃ 、3.04 MPa 条件下, 反应 70.0h, 以48%的产率得到2',3'-二脱氧肌苷参考文献:名称:Antonov; Konstantinova; Miroshnikov, Nucleosides and Nucleotides, 1998, vol. 17, # 1-3, p. 153 - 159摘要:DOI:
文献信息
-
[EN] SPIROCYCLIC HETEROCYCLE COMPOUNDS USEFUL AS HIV INTEGRASE INHIBITORS<br/>[FR] COMPOSÉS HÉTÉROCYCLIQUES SPIROCYCLIQUES UTILES COMME INHIBITEURS DU VIH申请人:MERCK SHARP & DOHME公开号:WO2016094198A1公开(公告)日:2016-06-16The present invention relates to Spirocyclic Heterocycle Compounds of Formula (I): (I) and pharmaceutically acceptable salts thereof, wherein A, B, X, R1, R2, R3 and R4 are as defined herein. The present invention also relates to compositions comprising at least one Spirocyclic Heterocycle Compound, and methods of using the Spirocyclic Heterocycle Compounds for treating or preventing HIV infection in a subject.
-
[EN] METALLOENZYME INHIBITOR COMPOUNDS<br/>[FR] COMPOSÉS INHIBITEURS DE MÉTALLOENZYMES
-
Integrase inhibitors申请人:Cai R. Zhenhong公开号:US20080058315A1公开(公告)日:2008-03-06Tricyclic compounds, protected intermediates thereof, and methods for inhibition of HIV-integrase are disclosed.三环化合物,其受保护的中间体,以及用于抑制HIV整合酶的方法被披露。
-
3-Aminocyclopentanecarboxamides as modulators of chemokine receptors申请人:Xue Chu-Biao公开号:US20060004018A1公开(公告)日:2006-01-05The present invention is directed to compounds of Formula I: which are modulators of chemokine receptors. The compounds of the invention, and compositions thereof, are useful in the treatment of diseases related to chemokine receptor expression and/or activity.
-
[EN] DERIVATIVES OF AMANITA TOXINS AND THEIR CONJUGATION TO A CELL BINDING MOLECULE<br/>[FR] DÉRIVÉS DE TOXINES D'AMANITES ET LEUR CONJUGAISON À UNE MOLÉCULE DE LIAISON CELLULAIRE申请人:HANGZHOU DAC BIOTECH CO LTD公开号:WO2017046658A1公开(公告)日:2017-03-23Derivatives of Amernita toxins of Formula (I), wherein, formula (a) R 1, R 2, R 3, R 4, R 5, R 6, R 7, R 8, R 9, R 10, X, L, m, n and Q are defined herein. The preparation of the derivatives. The therapeutic use of the derivatives in the targeted treatment of cancers, autoimmune disorders, and infectious diseases.
表征谱图
-
氢谱1HNMR
-
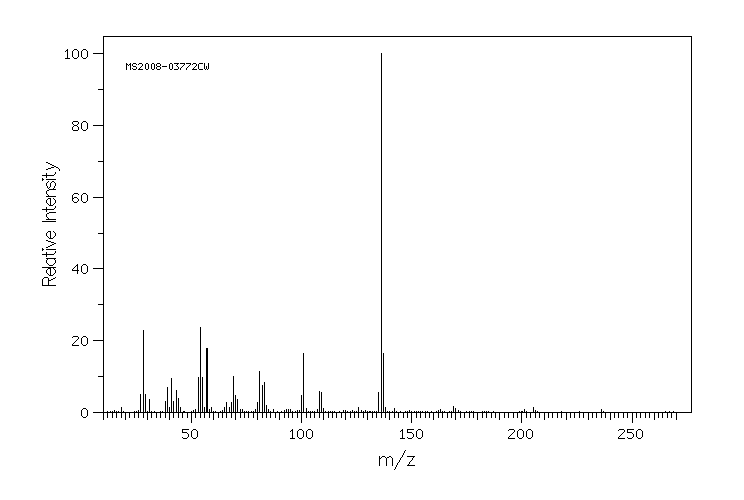
质谱MS
-
碳谱13CNMR
-
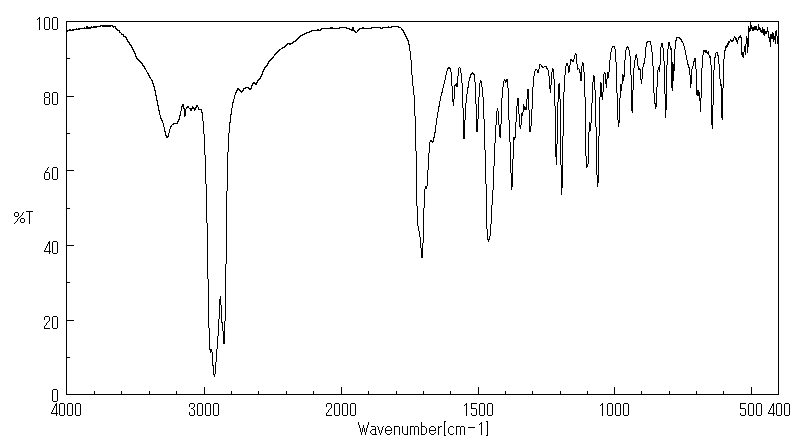
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息