久洛尼定 | 479-59-4
中文名称
久洛尼定
中文别名
多洛尼定;2,3,6,7-四氢-1H,5H-苯并[i,j]喹嗪;久洛利定
英文名称
julolidine
英文别名
2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinoline;2,3,6,7-tetrahydro-1H,5H-benzo[ij]quinolizine;1-azatricyclo[7.3.1.05,13]trideca-5(13),6,8-triene
CAS
479-59-4
化学式
C12H15N
mdl
MFCD00006917
分子量
173.258
InChiKey
DZFWNZJKBJOGFQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:34-36 °C(lit.)
-
沸点:170-173°C 31mm
-
密度:1.0030
-
闪点:>230 °F
-
溶解度:氯仿(微溶)、乙酸乙酯(微溶)
-
稳定性/保质期:
按规格使用和贮存,不会发生分解,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:13
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:3.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
TSCA:Yes
-
安全说明:S24/25
-
危险类别码:R52/53
-
WGK Germany:3
-
海关编码:2933990090
-
危险品运输编号:NONH for all modes of transport
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:密封保存,并置于通风干燥处,避免与其它氧化物接触。
SDS
模块 1. 化学品
1.1 产品标识符
: Julolidine
产品名称
1.2 鉴别的其他方法
2,3,6,7-Tetrahydro-1H,5H-benzo[ij]quinolizine
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
急性水生毒性 (类别 2)
2.2 GHS 标记要素,包括预防性的陈述
象形图 无
警示词 无
危险申明
H401 对水生生物有毒。
警告申明
预防措施
P273 避免释放到环境中。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: 2,3,6,7-Tetrahydro-1H,5H-benzo[ij]quinolizine
别名
: C12H15N
分子式
: 173.25 g/mol
分子量
组分 浓度或浓度范围
2,3,6,7-Tetrahydro-1H,5H-benzo[ij]quinolizine
<=100%
化学文摘登记号(CAS 479-59-4
No.) 207-535-0
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。
6.2 环境保护措施
如能确保安全,可采取措施防止进一步的泄漏或溢出。 不要让产品进入下水道。
一定要避免排放到周围环境中。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
建议的贮存温度: 2 - 8 °C
充气操作和储存
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所选择身体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
颜色: 灰白色或米色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 34 - 36 °C - lit.
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
113 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
辛醇--水的分配系数的对数值: 3.064
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
对水生生物有毒。
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
化学性质
白色晶体。其熔点为34-36℃(97%商品),而溴氢酸盐的熔点则在239-242℃之间。主要用于有机合成。
用途
久洛尼定是一类极为重要的氮杂环化合物,广泛应用于光化学工业中的荧光染料以及生物活性药物等相关产品的开发。
生产方法
将66.5克(0.5摩尔)四氢喹啉与400克1-溴-3-氯丙烷在150-160℃油浴中加热20小时,冷却后加入50毫升浓盐酸和500毫升水,并进行水蒸气蒸馏以除去过量的1-溴-3-氯丙烷。用40%氢氧化钠将剩余物调至碱性,然后用乙醚提取。乙醚提取液用水洗涤,经粒状氢氧化钠干燥后,蒸除乙醚并在减压下进行蒸馏。收集105-110℃(0.133kPa)的馏分,最终得到67-70克久洛尼定。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 3-(3,4-dihydroquinolin-1(2H)-yl)propan-1-ol 88014-20-4 C12H17NO 191.273 —— 2,3,6,7-tetrahydro-1H,5H-benzo quinolizin-1-ol 101359-27-7 C12H15NO 189.257 8-羟基久洛里定 8-hydroxyjulolidine 41175-50-2 C12H15NO 189.257 1,2,3,4-四氢喹啉 1,2,3,4-tetrahydroisoquinoline 635-46-1 C9H11N 133.193 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-amine-julolidine 59056-57-4 C12H16N2 188.272 —— 9-cyano-2,3,6,7-tetrahydro-1H,5H-benzo[ij]quinolizine 97315-60-1 C13H14N2 198.268 9-醛基久洛尼定 9-julolidinecarboxaldehyde 33985-71-6 C13H15NO 201.268 2,3,6,7-四氢-1H,5H-苯并(ij)喹嗪-9-甲醇 (2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-9-yl)-methanol 101077-18-3 C13H17NO 203.284 4-溴久洛尼定 9-bromo-2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinoline 70173-54-5 C12H14BrN 252.154 —— 9-formyl-2,3,6,7-tetrahydro-1H,5H-benzo[ij]quinolizine oxime 6310-83-4 C13H16N2O 216.283 —— p-nitrosojulolidine 33949-11-0 C12H14N2O 202.256 —— (E)-3-(2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-9-yl)acrylaldehyde 159923-42-9 C15H17NO 227.306 —— N-(2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-9-ylmethyl)-acetamide 101651-46-1 C15H20N2O 244.337 —— 2,3,6,7-tetrahydro-9-methoxy-1H,5H-benzo[ij]quinolizine 6403-55-0 C13H17NO 203.284 —— 1,2,6,7-tetrahydropyrido<3,2,1-i,j>quinolin-3(5H)-one 57369-31-0 C12H13NO 187.241 —— 2,3,6,7,2',3',6',7'-octahydro-1H,5H,1'H,5'H-[9,9']bi[pyrido[3,2,1-ij]quinolinyl] 33131-88-3 C24H28N2 344.5 —— 9-nitrojulolidine —— C12H14N2O2 218.255 —— 4-(2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-9-yl)benzaldehyde 1352574-11-8 C19H19NO 277.366 —— Bis(julolidinyl)ethane-1,2-dione 89375-11-1 C26H28N2O2 400.521 —— 9-(4-nitro-phenylazo)-2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinoline 32283-84-4 C18H18N4O2 322.367 —— [4-(2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-9-yl)benzylidene]propanedinitrile 201533-79-1 C22H19N3 325.413 —— (2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-9-yl)-ethenetricarbonitrile 58293-57-5 C17H14N4 274.325 —— 4-(2,3,6,7-Tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-9-yl)-3-chlorocyclobut-3-ene-1,2-dione 159148-48-8 C16H14ClNO2 287.746 —— 5-(2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-9-yl)thiophene-2-carbaldehyde 1352574-14-1 C17H17NOS 283.394 —— 4-(2,3,6,7-Tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-9-yl)-3-hydroxycyclobut-3-ene-1,2-dione 159148-50-2 C16H15NO3 269.3 —— 1-(2,3,6,7-tetrahydro-1H,5H-benzo[ij]quinolizin-9-yl)-2-(2-carboxybenzamido)ethanone 405168-04-9 C22H22N2O4 378.428 —— {[5-(2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-9-yl)thiophen-2-yl]methylidene}propanedinitrile 1352574-07-2 C20H17N3S 331.441 —— 1-(2,3,6,7-tetrahydro-1H,5H-benzo[ij]quinolizin-9-yl)-2-phthalimidoethanone 405168-03-8 C22H20N2O3 360.412 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:Preparation and Properties of some Substituted Julolidines摘要:DOI:10.1021/jo50009a015
-
作为产物:描述:参考文献:名称:Rindfusz; Harnack, Journal of the American Chemical Society, 1920, vol. 42, p. 1723摘要:DOI:
-
作为试剂:描述:久洛尼定 、 三氯氧磷 、 N,N-二甲基苯胺 、 N,N-二甲基甲酰胺 在 氮 、 Isopropanol carbon dioxide 、 三氯氧磷 、 久洛尼定 、 ice 、 水 、 sodium acetate 、 aldehyde 、 甲烷 、 water ethanol 作用下, 反应 18.42h, 以to give 21.2 g (96%) of 9-JA as light yellow needles, mp 81° C.-82° C. [lit. 83° C., J. Org. Chem., 17, 1281 (1952)]的产率得到9-醛基久洛尼定参考文献:名称:Process of forming hologram and polymeric holographic recording medium摘要:本发明揭示了一类从环状酮和三环氨基醛衍生的光聚合组合物敏化剂。其中一种优选化合物为环戊酮,2.5-双[(2,3,6,7-四氢-1H,5H-苯并[i,j]喹啉-9-基)亚甲基]- ##STR1##公开号:US05096790A1
文献信息
-
Direct <i>para</i>-C–H heteroarylation of anilines with quinoxalinones by metal-free cross-dehydrogenative coupling under an aerobic atmosphere作者:Jun Xu、Lin Huang、Lei He、Chenfeng Liang、Yani Ouyang、Jiabin Shen、Min Jiang、Wanmei LiDOI:10.1039/d1gc01899j日期:——Herein, a green and efficient metal-free cross-dehydrogenative coupling (CDC) for the direct para-C–H heteroarylation of anilines with quinoxalinones has been described. This reaction is performed in H2O/DMSO (v/v = 2 : 1) using air as the sole oxidant. Various anilines (primary, secondary and tertiary amines) and quinoxalinones are well compatible, affording the corresponding products in moderate-to-good
-
LIGHT-ENABLED DRUG DELIVERY申请人:Virginia Commonwealth University公开号:US20140236071A1公开(公告)日:2014-08-21Conjugates are provided which comprise a membrane permeable drug linked to a moiety that is not membrane permeable. Attachment of the moiety that is not membrane permeable prevents the drug from crossing cell membranes and entering cells. However, exposure to light either i) breaks the linkage, releasing the drug and allowing it to enter cells; or ii) converts the non-membrane permeable moiety to a membrane permeable form, allowing the entire conjugate to enter the cell, where the drug is released from the conjugate by cleavage. The membrane permeable drugs are thus delivered to cells at locations of interest, e.g. cancer cells in a tumor, in a temporally and spatially controlled manner.提供了一种缀合物,该缀合物包括与一个不可透过细胞膜的基团相连的可透过细胞膜的药物。不可透过细胞膜的基团的附着阻止了药物穿过细胞膜并进入细胞。然而,光照要么i)断裂这种连接,释放药物并允许其进入细胞;要么ii)将不可透过细胞膜的基团转化为可透过细胞膜的形式,允许整个缀合物进入细胞,在细胞中药物通过裂解从缀合物中释放。因此,可透过细胞膜的药物以时间和空间可控的方式被递送到感兴趣的位置,例如肿瘤中的癌细胞。
-
α-Cyanation of Aromatic Tertiary Amines using Ferricyanide as a Non-Toxic Cyanide Source作者:Alexander M. Nauth、Nicola Otto、Till OpatzDOI:10.1002/adsc.201500698日期:2015.11.16reaction of aromatic tertiary amines with potassium ferricyanide directly provides the useful α-amino nitriles. The inexpensive iron complex functions both as an oxidant and as a cyanide source. The presence of molecular oxygen speeds up the reaction which can be performed in aqueous tert-butanol or even in ethanol-based mixtures like Tequila. While amine cyanations usually employ highly toxic cyanide sources
-
Synthesis of C5-Allylindoles through an Iridium-Catalyzed Asymmetric Allylic Substitution/Oxidation Reaction Sequence of <i>N</i>-Alkyl Indolines作者:Jiamin Lu、Ruigang Xu、Haixia Zeng、Guofu Zhong、Meifang Wang、Zhigang Ni、Xiaofei ZengDOI:10.1021/acs.orglett.1c00810日期:2021.5.7Iridium/Brønsted acid cooperative catalyzed asymmetric allylic substitution reactions at the C5 position of indolines have been reported for the first time. The highly efficient protocol allows rapid access to various C5-allylated products in good to high yields (48–97%) and enantioselectivities (82% to >99% ee) with wide functional group tolerance. The transformations allow not only the formation
-
Synthesis of arylbromides from arenes and <i>N</i>-bromosuccinimide (NBS) in acetonitrile — A convenient method for aromatic bromination作者:Eli Zysman-Colman、Karla Arias、Jay S. SiegelDOI:10.1139/v08-176日期:2009.2
Regioselective and chemoselective electrophilic bromination of a wide series of activated arenes using N-bromosuccinimide (NBS) in acetonitrile occurs readily. Environmentally friendly conditions, large substrate scope, and ease of synthesis enhance the utility of this method over other electrophilic bromination conditions.
表征谱图
-
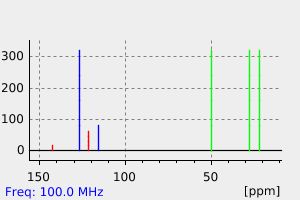
氢谱1HNMR
-
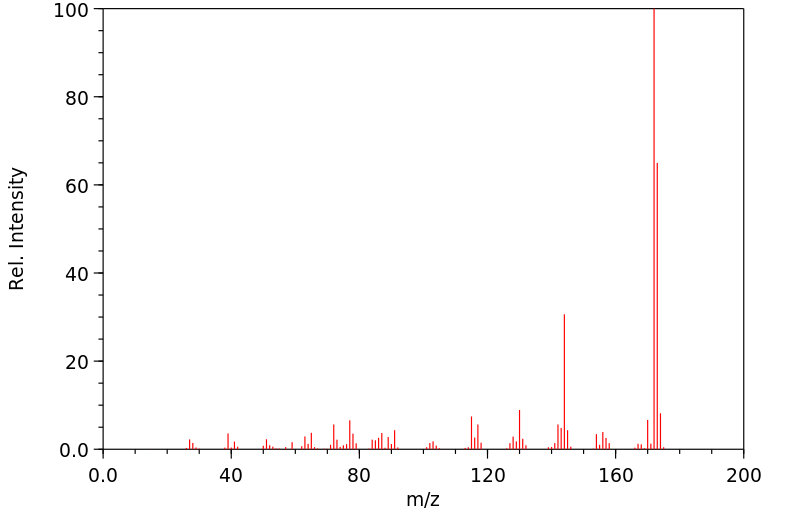
质谱MS
-
碳谱13CNMR
-
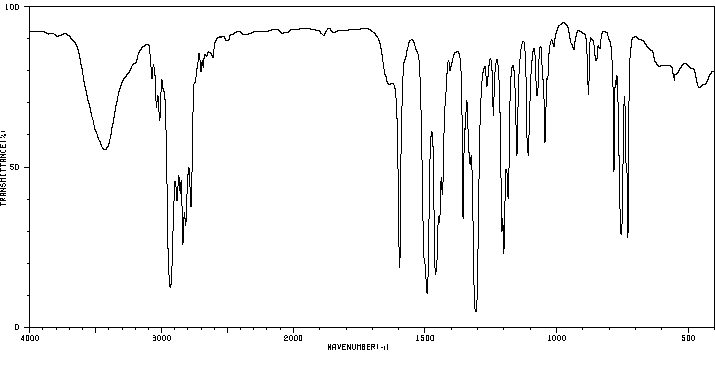
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43