乙酸乙酯 | 141-78-6
中文名称
乙酸乙酯
中文别名
醋酸乙酯;醋酸乙脂;EAC
英文名称
ethyl acetate
英文别名
Acetic acid ethyl ester;etOAc;AcOEt
CAS
141-78-6
化学式
C4H8O2
mdl
MFCD00009171
分子量
88.1063
InChiKey
XEKOWRVHYACXOJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:−84 °C(lit.)
-
沸点:76.5-77.5 °C(lit.)
-
密度:0.902 g/mL at 25 °C(lit.)
-
蒸气密度:3 (20 °C, vs air)
-
闪点:26 °F
-
溶解度:与乙醇、丙酮、乙醚和苯混溶。
-
最大波长(λmax):λ: 256 nm Amax: ≤1.00λ: 275 nm Amax: ≤0.05λ: 300 nm Amax: ≤0.03λ: 325-400 nm Amax: ≤0.005
-
介电常数:23.0(Ambient)
-
暴露限值:TLV-TWA 400 ppm (~1400 mg/m3) (ACGIH, MSHA, and OSHA); IDLH 10,000 ppm (NIOSH).
-
LogP:0.68-0.73 at 20-25℃
-
物理描述:Ethyl acetate appears as a clear colorless liquid with a fruity odor. Flash point 24°F. Less dense than water. Vapors heavier than air.
-
颜色/状态:Clear, volatile
-
气味:CHARACTERISTIC ETHER-LIKE ODOR REMINISCENT OF PINEAPPLE.
-
味道:Pleasant taste when diluted
-
蒸汽密度:3.04 (NTP, 1992) (Relative to Air)
-
蒸汽压力:93.2 mm Hg at 25 °C
-
亨利常数:Henry's Law constant = 1.34X10-4 atm-cu m/mole at 25 °C
-
大气OH速率常数:1.60e-12 cm3/molecule*sec
-
稳定性/保质期:
-
无色透明液体,能与氯仿、醇、丙酮及醚混溶;25℃时10ml水中可溶解1ml本品。温度升高会形成二元共沸混合物,与水形成的共沸混合物沸点为70.4℃,含水量6.1%(质量),与乙醇形成的共沸混合物沸点为71.8℃;与7.8%的水和9.0%的乙醇形成三元共沸混合物的沸点为70.2℃。该物质具有挥发性且易燃,带有水果香味。水分会使其缓慢分解并呈现酸性反应。易燃蒸汽与空气可形成爆炸性混合物,其爆炸极限为2.2%-11.2%(体积)。
-
化学性质:乙酸乙酯易于水解,在常温下有少量水存在时也会逐渐生成乙酸和乙醇。添加微量酸或碱能加速水解反应进程。此外,它还能发生醇解、氨解、酯交换以及还原等一般的酯类反应。在金属钠的催化作用下,会自行缩合形成3-羟基-2-丁酮或乙酰乙酸乙酯;与Grignard试剂反应生成酮,并进一步得到叔醇。该物质对热相对稳定,在290℃加热8~10小时不会发生改变。当通过红热的铁管时,会分解成乙烯和乙酸;在300~350℃加热锌粉时则分解为氢、一氧化碳、二氧化碳、丙酮及乙烯;而在360℃下经脱水的氧化铝处理后,可分解产生水、乙烯、二氧化碳与丙酮。紫外线照射下分解生成约55%的一氧化碳、14%的二氧化碳和31%的氢或甲烷等可燃性气体。在与臭氧反应时,则会生成乙醛和乙酸。气态卤化氢与乙酸乙酯反应,生成卤代乙烷及乙酸,其中碘化氢最为易反应;氯化氢在常温下需加压才可能发生分解,在150℃加热与五氯化磷一起使用时则生成氯乙烷和乙酰氯。此外,它还能与金属盐类形成各种结晶复合物,这些复合物溶于无水乙醇而不溶于乙酸乙酯,并且遇水容易水解。
-
稳定性:稳定 [23]
-
避免接触物质:强氧化剂、碱类、酸类
-
聚合风险:不聚合 [25]
-
-
自燃温度:427 °C
-
分解:When heated to decomposition it emits acrid smoke and irritating fumes.
-
粘度:0.423 mPa.s at 25 °C
-
燃烧热:2238.1 kJ/mol
-
汽化热:35.60 kJ/mol at 25 °C
-
表面张力:24 DYNES/CM AT 20 °C
-
电离电位:10.01 eV
-
气味阈值:Odor Threshold Low: 6.4 [mmHg]; Odor Threshold High: 50.0 [mmHg]; Detection odor threshold from AIHA (mean = 18 ppm)
-
折光率:Index of refraction = 1.3723 at 20 °C/D
-
保留指数:577 ;596 ;598 ;609 ;578 ;581 ;581 ;599 ;601 ;581 ;581 ;601 ;597 ;600 ;600 ;600 ;602 ;620 ;600 ;599 ;602 ;600 ;594 ;624 ;603 ;603 ;600 ;578.95 ;597.3 ;599.3 ;600.8 ;604.4 ;605.1 ;609.7 ;611.7 ;563 ;600 ;587 ;589 ;600 ;601 ;600 ;600 ;595 ;577 ;607 ;600 ;577 ;607 ;613 ;572 ;579 ;580 ;590 ;642 ;594 ;595.7 ;596 ;611 ;611 ;600 ;600 ;592 ;592 ;592 ;602 ;571 ;602 ;590 ;593 ;594 ;593 ;589 ;592 ;587 ;590 ;591 ;594 ;592 ;594 ;597 ;602.3 ;595 ;598.8 ;598 ;612 ;592 ;628 ;595 ;601 ;596 ;600 ;600 ;601.82 ;600 ;604.7 ;609 ;602 ;598 ;597 ;602 ;583 ;592 ;604 ;600 ;601 ;602 ;603 ;580 ;590 ;600 ;596 ;598 ;612 ;590 ;600 ;595 ;600 ;600 ;590 ;594 ;595 ;600 ;600 ;600 ;600 ;600 ;595 ;595 ;595 ;605 ;604 ;593 ;600 ;611 ;616 ;600 ;596 ;605 ;605 ;607 ;615 ;603 ;608 ;610 ;598.8 ;612 ;603.8
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:6
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
ADMET
代谢
当前研究旨在量化F344大鼠和叙利亚仓鼠上呼吸道(URT)中吸入乙酸乙酯的代谢程度。在恒定速度单向流动条件下,测量了这些物种在手术隔离的URT中的乙酸乙酯沉积量。通过基于简单的静脉平衡方法的数学建模和直接比较未经处理的和被羧酸酯酶抑制的动物之间的沉积效率,估计了代谢程度。在大鼠URT中,乙酸乙酯的沉积效率平均在10%到35%之间,而在仓鼠中为36%到72%。羧酸酯酶抑制降低了两种物种的沉积。建模工作和未经处理与抑制动物之间的直接比较表明,大量沉积的乙酸乙酯在两种物种的URT中被代谢,且仓鼠的代谢程度更为显著。具体来说,在大鼠URT中,40-65%的沉积乙酸乙酯被代谢,而在仓鼠中这一比例为63-90%。这种首次通过代谢(i)增加了URT的沉积效率;(ii)导致URT组织中代谢物水平升高;并且(iii)减少了URT中可用于吸收进入血液的母体乙酸乙酯的数量。
... The current study was aimed at quantitating the extent of metabolism of inspired ethyl acetate in the upper respiratory tract (URT) of the F344 rat and Syrian hamster. Ethyl acetate deposition was measured in the surgically isolated URT of these species under constant velocity unidirectional flow conditions. The degree of metabolism was estimated by mathematic modeling based on a simple venous-equilibration approach and by direct comparison of deposition efficiencies in naive and carboxylesterase-inhibited animals. Ethyl acetate deposition efficiencies averaged between 10 and 35% in the rat URT and 36 and 72% in the hamster. Carboxylesterase inhibition decreased deposition in both species. Both the modeling efforts and the direct comparisons between naive and inhibited animals indicated that significant amounts of the deposited ethyl acetate were metabolized in the URT of both species with the extent of metabolism being more pronounced in the hamster. Specifically, 40-65% of the deposited ethyl acetate was metabolized in the URT of the rat compared to 63-90% in the hamster. This first-pass metabolism (i) increased URT deposition efficiencies; (ii) led to production of high metabolite levels in URT tissues; and (iii) decreased the amount of parent ethyl acetate available for absorption into the bloodstream in the URT.
来源:Hazardous Substances Data Bank (HSDB)
代谢
由于非特异性酯酶的丰富存在,人们可能会预期常见的溶剂乙酸乙酯(EtAc)会在体内被水解为乙醇(EtOH)。这样,就有可能通过暴露于EtAc蒸气后展示EtOH的积累。初步研究表明,在大鼠血液中孵化37°C时,EtAc确实会被水解为EtOH,其半衰期大约为65分钟。分析是通过气相色谱进行的。为了在体内研究这个反应,大鼠被戊巴比妥麻醉,并在股动脉中插入导管。EtAc以25%的溶液(1.6 g/kg)玉米油的形式注射到腹腔中,并定期抽取血液样本。在体内的水解非常迅速,估计半衰期为5-10分钟。随后通过让麻醉大鼠通过气管导管暴露于几种浓度的EtAc蒸气来进行吸入研究。当EtAc浓度增加到2000 ppm以上时,EtAc的吸收超过了EtOH的氧化,导致血液中EtOH的积累。尽管血液中EtOH浓度在5小时内稳步增加到超过0.10 g/100 mL,但EtAc始终保持在0.01 g/100 mL以下,并且在整个实验过程中没有变化,这再次表明了迅速的水解。数据表明,如果EtAc的环境浓度足够高,在暴露于EtAc期间EtOH将会积累。
Because of the abundance of nonspecific esterases, one might expect the common solvent ethyl acetate (EtAc) to be hydrolyzed to ethyl alcohol (EtOH) in vivo. It would then be possible to demonstrate EtOH accumulation following exposure to EtAc vapor. Preliminary studies showed that rat blood incubated at 37 °C does hydrolyze EtAc to EtOH, with a half-time of approximately 65 min. Analyses were done by gas chromatography. To study this reaction in vivo, rats were anesthetized with pentobarbital, and cannulae were inserted into the femoral arteries. EtAc was injected ip as a 25% () solution in corn oil (1.6 g/kg) and blood samples were drawn periodically. Hydrolysis was very rapid in vivo, with a half-time estimated at 5-10 min. Inhalation studies were then carried out by exposing anesthetized rats to several concentrations of EtAc vapor via an endotracheal tube. When EtAc concentrations were increased above 2000 ppm, EtAc absorption exceeded EtOH oxidation, leading to an accumulation of EtOH in the blood. Although blood EtOH concentrations increased steadily to over 0.10 g/100 mL in 5 hr, EtAc remained consistently below 0.01 g/100 mL and did not change throughout the course of the experiment, again indicating rapid hydrolysis. The data indicate that EtOH will accumulate during exposure to EtAc if the ambient concentration of EtAc is sufficiently high.
来源:Hazardous Substances Data Bank (HSDB)
代谢
... Ethyl acetate ... metabolism produces corresponding ethyl alcohol & is partly excreted in exhaled air & urine & partly metabolized.
来源:Hazardous Substances Data Bank (HSDB)
代谢
在大鼠的代谢研究中,发现了一个大约2000 ppm的无效应水平。在更高水平下,乙酸乙酯的水解速率似乎超过了乙醇的氧化速率,导致其在血管系统中积累。另外,当以1.6 g/kg的剂量腹腔注射时,迅速水解为醋酸和乙醇。对雄性大鼠连续8天腹腔注射1 mL/kg,显著增加了血液中丙酮酸和乳酸的含量,并提高了糖酵解酶的活性。
Metabolic studies in the rat have revealed an approximate 2000 ppm no-effect level. At higher levels, the rate of hydrolysis of ethyl acetate appeared to exceed ethanol oxidation, leading to its accumulation in the vascular system. Also, when it was injected intraperitoneally at 1.6 g/kg, hydrolysis to acetic acid and ethanol occurred rapidly. Intraperitoneal injections of 1 mL/kg to male rats for 8 days increased the blood pyruvic and lactic acid content considerably and also elevated the glycolytic enzymatic activity.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
识别和使用:乙酸乙酯是一种无色液体,具有类似胶水或指甲油的气味,用作工业溶剂。乙酸乙酯用作化学反应的溶剂。由于其气味,它常用于化妆品中,其气味与指甲油相关联。此外,它还用于糖果、香水和水果中,因为它挥发速度快,只留下香水在皮肤上的香味。乙酸乙酯是对昆虫收藏家有效的毒药,因为其蒸气是呼吸道刺激物,其蒸气可以迅速杀死昆虫而不破坏它,使其完整无缺以供研究。人类暴露和毒性:短期暴露于高浓度的乙酸乙酯首先会导致眼睛、鼻子和喉咙的刺激,随后出现头痛、恶心、呕吐、嗜睡和昏迷。高浓度会导致中枢神经系统抑制以及肝脏和肾脏的充血。慢性中毒被描述为产生贫血、白细胞增多(暂时性白细胞计数增加)和混浊肿胀以及脂肪变性。在正在建设中的设施中训练后抱怨喘息咳嗽、鼻炎或呼吸困难的跑步者进行了评估。调查发现乙酸乙酯和甲苯的水平足以满足联邦指南,但显然足以引起运动员的症状。其致癌性质尚不清楚。在早期年份暴露于300 mL/立方米的乙酸乙酯浓度的工人,在调查时暴露于16 mL/立方米的水平,发现精子质量正常。动物研究:在动物中,浓度超过5000 ppm时具有麻醉效果。家兔每天1小时,连续40天暴露于4450 ppm,出现贫血伴白细胞增多,以及肝脏和肾脏损伤。雄性大鼠经灌胃高剂量(3600 mg/kg/天)的乙酸乙酯,显示出显著的毒性效应,导致体重和器官重量下降,食物消耗量减少。雌性大鼠暴露于高剂量,上述参数与对照组相比略有但无统计学意义下降。大鼠每天6小时,每周5天,连续13周通过吸入暴露于750和1500 ppm的乙酸乙酯,在暴露期间对意外听觉刺激的行为反应减弱,并似乎具有急性镇静作用。暴露会话结束30分钟后没有急性中毒的迹象。暴露于750和1500 ppm的大鼠体重、体重增加、食物消耗和食物效率降低,但在4周内完全或部分恢复。在350 ppm的雄性大鼠中观察到体重增加和食物效率的降低。亚慢性暴露的主要行为效应是1500 ppm雌性的运动活动减少,这种效应在4周恢复期后不存在。所有其他功能观察电池和运动活动参数均未受影响,神经组织未观察到病理学变化。总之,没有证据表明亚慢性暴露至1500 ppm的乙酸乙酯在大鼠中产生了任何持久的神经毒性效应。乙酸乙酯是酿酒酵母中诱导非整倍体的强诱导剂,但在鼠伤寒沙门氏菌的突变性试验中呈阴性。溶剂在体内对中国仓鼠的微核试验结果为阴性。体内乙酸乙酯水解为乙酸和乙醇的速度很快。生态毒性研究:普通印度鲶鱼(Heteropneustes fossilus)暴露于170 ppm的乙酸乙酯3、6、12、48和96小时,引起了碳水化合物代谢的显著变化。在3、48和96小时,肝糖原水平显著下降,但在任何暴露期间肌肉糖原含量都没有明显变化。在所有时间间隔都出现高血糖。在3、6、48和96小时,血液丙酮酸水平升高。在3和96小时出现高乳酸血症,但在6和12小时出现低乳酸血症。碳水化合物代谢受损可能是乙酸乙酯的毒性作用的原因。
IDENTIFICATION AND USE: Ethyl Acetate is a colorless liquid with a smell similar to glue or nail polish that is used as an industrial solvent. Ethyl Acetate is used as a solvent for chemical reactions. Because of its odor it is often used in cosmetics and its smell is associated with nail polishes. Additionally, it is used in confectionery, perfumes, and fruits because it evaporates at a fast rate, leaving but the scent of the perfume on the skin. Ethyl acetate is an effective poison for use in insect collector as its vapors are a respiratory tract irritant whose vapors can kill the insect quickly without destroying it, leaving it intact for study. HUMAN EXPOSURE AND TOXICITY: Short-term exposure to high levels of ethyl acetate results first in irritation of the eyes, nose and throat, followed by headache, nausea, vomiting, sleepiness, and unconsciousness. High concentrations can cause CNS depression and congestion of the liver and kidneys. Chronic poisoning has been described as producing anemia, leucocytosis (transient increase in the white blood cell count), and cloudy swelling, and fatty degeneration. Runners were evaluated after complaining of wheezing coughing, rhinitis, or shortness of breath after practicing in a facility under construction. Investigation revealed levels of ethyl acetate and toluene low enough to meet federal guidelines but apparently sufficient to cause symptoms in the athletes. Its carcinogenic properties are not known. Workers who in earlier years had been exposed to ethyl acetate concentrations of 300 mL/cu m and who were exposed at the time of the investigation to 16 mL/cu m were found to have normal sperm quality. ANIMAL STUDIES: In animals it has a narcotic effect at concentrations of over 5000 ppm. Repeated exposures of rabbits to 4450 ppm for 1 hr daily for 40 days resulted in anemia with leukocytosis, and damage to liver and kidneys. Male rats exposed to a high dose (3600 mg/kg/day) of ethyl acetate by gavage showed significant toxic effects, which resulted in depressed body and organ weights, and depressed food consumption. Female rats exposed to the high dose showed slight but non-significant depression of above parameters compared with controls. Exposure of rats to 750 and 1500 ppm ethyl acetate via inhalation for 6 hr per day, 5 days per week for 13 weeks, diminished behavioral responses to unexpected auditory stimuli during the exposure session and appeared to have an acute sedative effect. There were no signs of acute intoxication 30 min after exposure sessions ended. Rats exposed to 750 and 1500 ppm had reduced body weight, body weight gain, feed consumption, and feed efficiency, which fully or partially recovered within 4 weeks. Reductions in body weight gain and feed efficiency were observed in male rats exposed to 350 ppm. The principal behavioral effect of subchronic exposure was reduced motor activity in the 1500 ppm females, an effect that was not present after the 4-week recovery period. All other functional observation battery and motor activity parameters were unaffected, and no pathology was observed in nervous system tissues. In conclusion, there was no evidence that subchronic exposure up to 1500 ppm ethyl acetate produced any enduring neurotoxic effects in rats. Ethyl acetate is strong inducer of aneuploidy in the yeast Saccaromyces cerevisiae, but was negative for mutagenicity in Salmonella typhimurium assays. The solvent yielded negative result in the micronucleus assay in Chinese hamsters in vivo. In vivo hydrolysis of ethyl acetate to acetic acid and ethanol occurred rapidly. ECOTOXICITY STUDIES: Exposure of the common indian catfish (Heteropneustes fossilus) to 170 ppm of ethyl acetate for 3, 6, 12, 48, and 96 hr induced marked changes in carbohydrate metabolism. Hepatic glycogen levels declined significantly at 3, 48, and 96 hr, but there was no marked alteration in muscle glycogen content at any of the exposure periods. Hyperglycemia occurred at all time intervals. Blood pyruvate levels were elevated at 3, 6, 48, and 96 hr. Hyperlacticemia resulted at 3 and 96 hr, but hypolacticemia occurred at 6 and 12 hr. Impairment of carbohydrate metabolism might be responsible for the toxic action of ethyl acetate.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
该物质可以通过吸入其蒸气被身体吸收。
The substance can be absorbed into the body by inhalation of its vapour.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
吸入,摄入,皮肤和/或眼睛接触
inhalation, ingestion, skin and/or eye contact
来源:The National Institute for Occupational Safety and Health (NIOSH)
毒理性
眼睛、皮肤、鼻子、喉咙刺激;麻醉;皮炎
irritation eyes, skin, nose, throat; narcosis; dermatitis
来源:The National Institute for Occupational Safety and Health (NIOSH)
毒理性
喉咙痛。咳嗽。头痛。困倦。
Sore throat. Cough. Headache. Drowsiness.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
吸收、分配和排泄
乙基醋酸产生毒性的主要吸收途径是吸入。
... The primary route of absorption responsible for the toxicity of ethyl acetate is inhalation.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
职业暴露等级:A
-
职业暴露限值:TWA: 400 ppm (1400 mg/m3)
-
TSCA:Yes
-
危险等级:3
-
立即威胁生命和健康浓度:2,000 ppm [10% LEL]
-
危险品标志:F
-
安全说明:S16,S26,S33,S36/37,S45,S7
-
危险类别码:R66,R36,R67,R11
-
WGK Germany:1
-
海关编码:2915310000
-
危险品运输编号:UN 1173 3/PG 2
-
危险类别:3
-
RTECS号:AH5425000
-
包装等级:II
-
危险标志:GHS02,GHS07
-
危险性描述:H225,H319,H336
-
危险性防范说明:P210,P305 + P351 + P338,P370 + P378,P403 + P235
-
储存条件:储存注意事项:储存于阴凉、通风的库房,远离火种、热源,库温不宜超过37℃。保持容器密封,并与氧化剂、酸类、碱类分开存放,切忌混储。采用防爆型照明和通风设施,禁止使用易产生火花的机械设备和工具。储区应配备泄漏应急处理设备和合适的收容材料。
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 乙酸酐 acetic anhydride 108-24-7 C4H6O3 102.09 乙酸甲酯 Methyl acetate 79-20-9 C3H6O2 74.0794 甲乙酐 Acetic formic anhydride 2258-42-6 C3H4O3 88.063 1,2-乙二醇单乙酸酯 2-hydroxyethyl acetate 542-59-6 C4H8O3 104.106 乙酸丙酯 1-Propyl acetate 109-60-4 C5H10O2 102.133 醋酸异丙酯 Isopropyl acetate 108-21-4 C5H10O2 102.133 2-溴乙基乙酸酯 bromoethyl acetate 927-68-4 C4H7BrO2 167.002 甲酸乙酯 formic acid ethyl ester 109-94-4 C3H6O2 74.0794 丙酸乙酯 Ethyl propionate 105-37-3 C5H10O2 102.133 氟乙酸乙酯 ethyl 2-fluoroacetate 459-72-3 C4H7FO2 106.097 碘代醋酸乙酯 ethyl iodoacetae 623-48-3 C4H7IO2 214.003 溴乙酸乙酯 ethyl bromoacetate 105-36-2 C4H7BrO2 167.002 氯乙酸乙酯 chloroacetic acid ethyl ester 105-39-5 C4H7ClO2 122.551 乙酸乙烯酯 vinyl acetate 108-05-4 C4H6O2 86.0904 乙酸丁酯 acetic acid butyl ester 123-86-4 C6H12O2 116.16 醋酸叔丁酯 acetic acid tert-butyl ester 540-88-5 C6H12O2 116.16 乙二醇二乙酸酯 ethylene glycol diacetate 111-55-7 C6H10O4 146.143 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 乙酸酐 acetic anhydride 108-24-7 C4H6O3 102.09 乙酸甲酯 Methyl acetate 79-20-9 C3H6O2 74.0794 甲乙酐 Acetic formic anhydride 2258-42-6 C3H4O3 88.063 乙酰氧基乙醛 acetoxyacetaldehyde 5371-49-3 C4H6O3 102.09 1,2-乙二醇单乙酸酯 2-hydroxyethyl acetate 542-59-6 C4H8O3 104.106 乙酸丙酯 1-Propyl acetate 109-60-4 C5H10O2 102.133 醋酸异丙酯 Isopropyl acetate 108-21-4 C5H10O2 102.133 乙酸烯丙酯 Allyl acetate 591-87-7 C5H8O2 100.117 乙醛酸乙酯 ethyl Glyoxylate 924-44-7 C4H6O3 102.09 2-氯乙酸乙酯 2-chloro-1-acetoxyethane 542-58-5 C4H7ClO2 122.551 乙酸炔丙酯 Propargyl acetate 627-09-8 C5H6O2 98.1014 甲酸乙酯 formic acid ethyl ester 109-94-4 C3H6O2 74.0794 丙酸乙酯 Ethyl propionate 105-37-3 C5H10O2 102.133 丙烯酸乙酯 ethyl acrylate 140-88-5 C5H8O2 100.117 溴乙酸乙酯 ethyl bromoacetate 105-36-2 C4H7BrO2 167.002 氯乙酸乙酯 chloroacetic acid ethyl ester 105-39-5 C4H7ClO2 122.551 碘代醋酸乙酯 ethyl iodoacetae 623-48-3 C4H7IO2 214.003 乙二醇甲醚乙酸酯 2-methoxyethyl acetate 110-49-6 C5H10O3 118.133 乙酸乙烯酯 vinyl acetate 108-05-4 C4H6O2 86.0904 乙二醇乙醚醋酸酯 2-Ethoxyethyl acetate 111-15-9 C6H12O3 132.159 3-羟基丙基乙酸酯 6-hydroxy-3-oxa-2-hexanone 36678-05-4 C5H10O3 118.133 乙酸丁酯 acetic acid butyl ester 123-86-4 C6H12O2 116.16 醋酸叔丁酯 acetic acid tert-butyl ester 540-88-5 C6H12O2 116.16 1-氯乙基乙酸酯 1-chloroethyl acetate 5912-58-3 C4H7ClO2 122.551 乙酸异丁酯 2-methylpropyl acetate 110-19-0 C6H12O2 116.16 乙二醇二乙酸酯 ethylene glycol diacetate 111-55-7 C6H10O4 146.143 丙烯酸甲酯(MA) acrylic acid methyl ester 96-33-3 C4H6O2 86.0904 —— crotyl acetate 628-08-0 C6H10O2 114.144 2-氯乙基2-氯乙酸酯 Chloro-acetic acid 2-chloro-ethyl ester 3848-12-2 C4H6Cl2O2 156.996 二乙二醇二乙酸酯 diethyleneglycol diacetate 628-68-2 C8H14O5 190.196 乙酸甲氧三甘酯 2-(2-(2-methoxyethoxy)ethoxy)ethyl acetate 3610-27-3 C9H18O5 206.239 2,2'-[氧基二(乙烷-2,1-二基氧基)]二乙基二乙酸酯 bis-[2-(2-acetoxy-ethoxy)-ethyl]-ether 22790-12-1 C12H22O7 278.302 3-氯丙基乙酸酯 3-Chloropropyl acetate 628-09-1 C5H9ClO2 136.578 3-(乙酰氧基)丙烷-1-硫醇 3-(acetyloxy)propane-1-thiol 26473-61-0 C5H10O2S 134.199 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:描述:参考文献:名称:吖啶橙半(氯化锌)盐作为路易斯酸-光氧化还原杂化催化剂用于生成 α-羰基自由基摘要:据报道,一种易于获得的有机-无机杂化催化剂用于α-卤代羰基化合物的还原断裂。稳健的杂化催化剂是 ZnCl 2 Lewis 酸和吖啶橙作为光活性有机染料的自稳定组合。已经使用光物理和电化学实验详细研究了这种混合催化剂的机械特性。一项系统的研究使我们能够发现合适的路易斯酸以有效地稳定α-卤代羰基化合物,从而降低标准有机染料范围内的还原电位。该策略通过引导光氧化还原循环通过氧化猝灭途径解决了脱卤氢化或烷基自由基同源偶联等问题。光活性有机染料和路易斯酸对应物之间的协同作用通过有效和受控地生成烷基自由基,使功能化与广泛的偶联伙伴成为可能,并作为昂贵的后过渡金属基光催化剂的合适替代品。为了证明这种协同催化体系的应用潜力,研究了四种不同的α-羰基溴的合成转化,具有广泛的底物范围。DOI:10.1002/adsc.202101053
-
作为产物:参考文献:名称:Birkinshaw; Charles; Raistrick, 1931, vol. 220, p. 355摘要:DOI:
-
作为试剂:参考文献:名称:10.1002/ejoc.202400387摘要:Iminosydnones (ImSyds) have renowned biological activities and attractive chemical properties for chemical biology. However, access to N6‐functionalized iminosydnones involves tricky and hazardous procedures. Thanks to an innovative mechanochemical strategy using 1,1’‐carbonyldiimidazole (CDI) as an activating agent, a straightforward synthesis of molsidomine and of a mesocarb analog, two bioactive iminosydnones, was achieved. The whole process, involving 4 linear steps, was carried out in the solid state by ball‐milling and with safe reagents, offering efficient and sustainable access to N‐carbonylated iminosydnones, in agreement with the principles of green chemistry.DOI:10.1002/ejoc.202400387
文献信息
-
[EN] AURORA KINASE MODULATORS AND METHOD OF USE<br/>[FR] MODULATEURS D'AURORA KINASE ET PROCÉDÉ D'UTILISATION申请人:AMGEN INC公开号:WO2009117157A1公开(公告)日:2009-09-24The present invention relates to chemical compounds having a general formula (I) wherein A1-5 and 7-8, D', L1, L2, R1, R3, R6-8, n and o are defined herein, and synthetic intermediates, which are capable of modulating the activity of Aurora kinase proteins and, thereby, influencing various disease states and conditions related to the activities of Aurora kinases. For example, the compounds are capable of influencing the process of cell cycle and cell proliferation to treat cancer and cancer-related diseases. The invention also includes pharmaceutical compositions, including the compounds, and methods of treating disease states related to the activity of Aurora kinase.本发明涉及具有一般式(I)的化合物,其中在此定义了A1-5和7-8,D',L1,L2,R1,R3,R6-8,n和o,以及合成中间体,这些化合物能够调节枢纽激酶蛋白的活性,从而影响与枢纽激酶活动相关的各种疾病状态和病况。例如,这些化合物能够影响细胞周期和细胞增殖过程,以治疗癌症和与癌症相关的疾病。该发明还包括包括这些化合物的药物组合物,以及治疗与枢纽激酶活性相关的疾病状态的方法。
-
[EN] NOVEL ANTIVIRAL COMPOUNDS<br/>[FR] NOUVEAUX COMPOSÉS ANTIVIRAUX申请人:UNIV LEUVEN KATH公开号:WO2014170368A1公开(公告)日:2014-10-23The present invention relates to a series of novel compounds and derivatives thereof, methods to prevent or treat viral infections by using the novel compounds, processes for their preparation, their use to treat or prevent viral infections and their use to manufacture a medicine to treat or prevent viral infections, preferably infections with viruses belonging to the family of the Togaviridae and more preferably infections with chikungunya virus (CHIKV).本发明涉及一系列新化合物及其衍生物,利用这些新化合物预防或治疗病毒感染的方法,以及它们的制备过程,用于治疗或预防病毒感染以及用于制造治疗或预防病毒感染的药物,最好是用于治疗属于Togaviridae家族的病毒,更好地是用于治疗寨卡病毒感染。
-
[EN] COMPOUNDS AND THEIR USE AS BACE INHIBITORS<br/>[FR] COMPOSÉS ET LEUR UTILISATION EN TANT QU'INHIBITEURS DE BACE申请人:ASTRAZENECA AB公开号:WO2016055858A1公开(公告)日:2016-04-14The present application relates to compounds of formula (I), (la), or (lb) and their pharmaceutical compositions/preparations. This application further relates to methods of treating or preventing Αβ-related pathologies such as Down's syndrome, β- amyloid angiopathy such as but not limited to cerebral amyloid angiopathy or hereditary cerebral hemorrhage, disorders associated with cognitive impairment such as but not limited to MCI ("mild cognitive impairment"), Alzheimer's disease, memory loss, attention deficit symptoms associated with Alzheimer's disease, neurodegeneration associated with diseases such as Alzheimer's disease or dementia, including dementia of mixed vascular and degenerative origin, pre-senile dementia, senile dementia and dementia associated with Parkinson's disease.本申请涉及式(I)、(Ia)或(Ib)的化合物及其药物组合物/制剂。本申请进一步涉及治疗或预防与Αβ相关的病理学,如唐氏综合症,β-淀粉样蛋白血管病,如但不限于脑淀粉样蛋白血管病或遗传性脑出血,与认知损害相关的疾病,如但不限于MCI(“轻度认知损害”),阿尔茨海默病,记忆丧失,与阿尔茨海默病相关的注意力缺陷症状,与疾病如阿尔茨海默病或痴呆症相关的神经退行性疾病,包括混合性血管性和退行性起源的痴呆,早老性痴呆,老年性痴呆和与帕金森病相关的痴呆的方法。
-
A General Proline‐Catalyzed Synthesis of 4,5‐Disubstituted <i>N</i> ‐Sulfonyl‐1,2,3‐Triazoles from 1,3‐Dicarbonyl Compounds and Sulfonyl Azide作者:Shanmugam Rajasekar、Pazhamalai AnbarasanDOI:10.1002/asia.201901015日期:2019.12.13An efficient proline-catalyzed synthesis of 4,5-disubstituted-N-sulfonyl-1,2,3-triazoles has been accomplished from 1,3-dicarbonyl compounds and sulfonyl azides. The developed reaction is suitable for various symmetrical and unsymmetrical 1,3-dicarbonyl compounds, tolerates various functional groups and affords 4,5-disubstituted-N-sulfonyl-1,2,3-triazoles in good yield with excellent regioselectivity
-
Rh(III)-Catalyzed Regio- and Chemoselective [4 + 1]-Annulation of Azoxy Compounds with Diazoesters for the Synthesis of 2<i>H</i>-Indazoles: Roles of the Azoxy Oxygen Atom作者:Zhen Long、Zhigang Wang、Danni Zhou、Danyang Wan、Jingsong YouDOI:10.1021/acs.orglett.7b00631日期:2017.6.2tandem C–H alkylation/intramolecular decarboxylative cyclization of azoxy compounds with diazoesters for the synthesis of 3-acyl-2H-indazoles is disclosed. The azoxy instead of the azo group enables a distinct approach for cyclative capture, leading to a [4 + 1]-annulation rather than a classic [4 + 2] manner. The azoxy oxygen atom is traceless after annulation, and further removal from the product is揭示了用Rh(III)催化的重氮化合物与重氮酸酯的串联C–H烷基化/分子内脱羧环化反应,可合成3-酰基-2 H-吲唑。取代偶氮基团的是偶氮氧基团,从而实现了一种独特的环化捕获方法,从而导致了[4 +1]环化,而不是经典的[4 + 2]方式。环氧化后,z氧基氧原子是无痕的,不需要进一步从产物中除去。该反应具有对不对称的乙氧基苯的完全区域选择性和单芳基二烯氧化物的相容性。
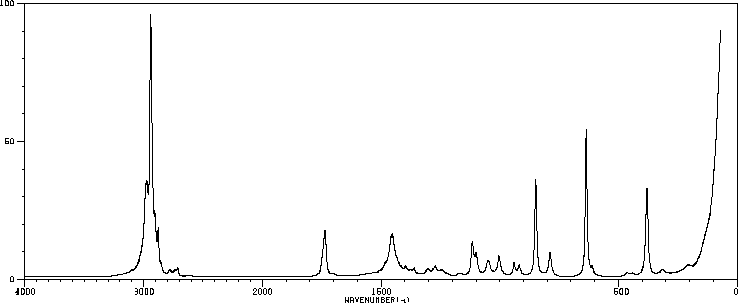
表征谱图
-
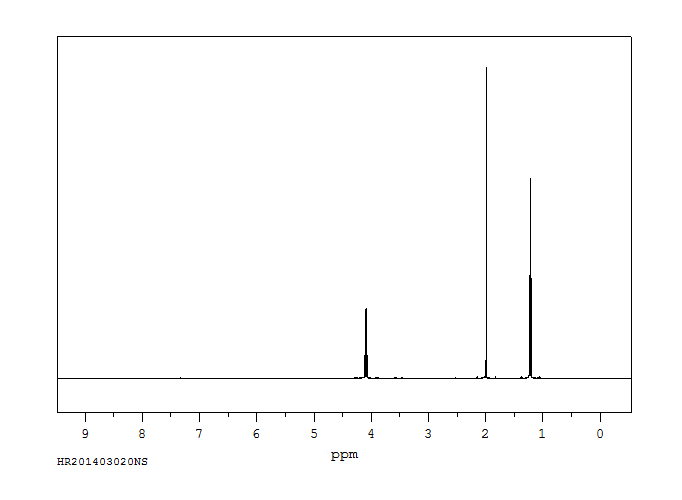
氢谱1HNMR
-
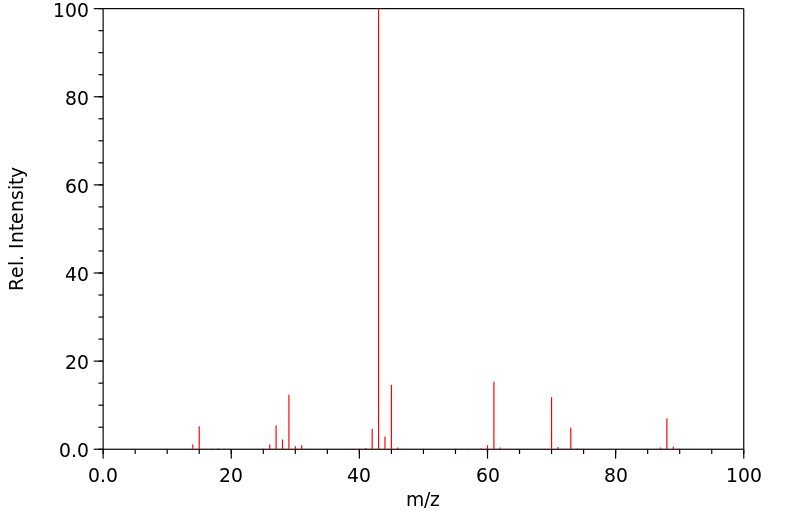
质谱MS
-
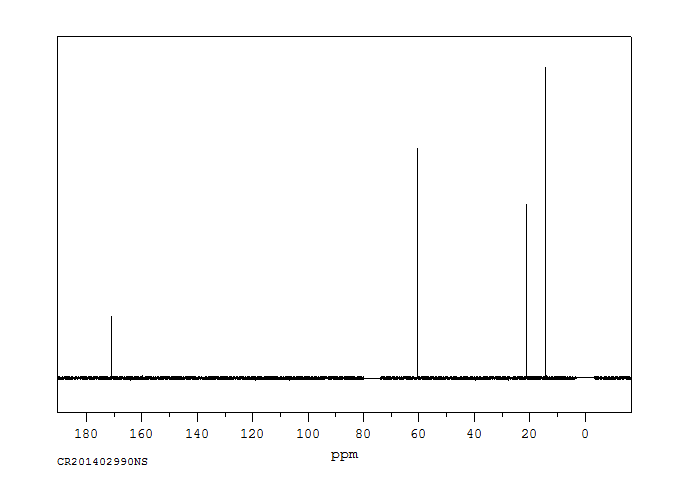
碳谱13CNMR
-
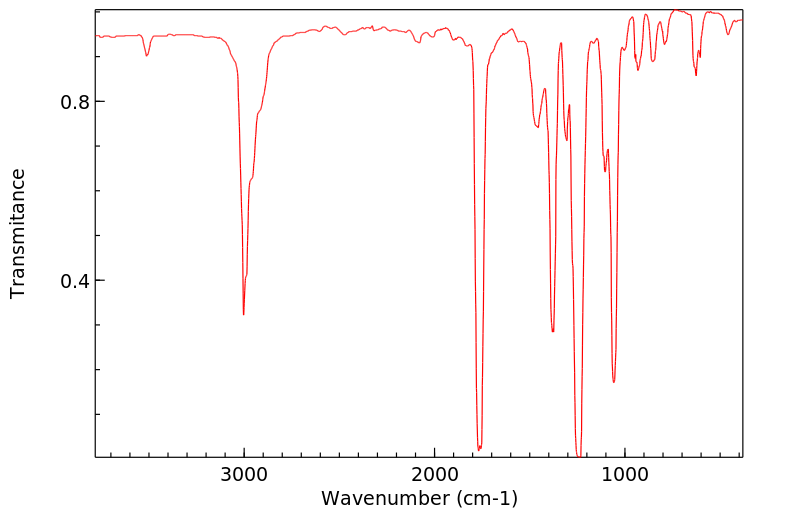
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸