乙酸甲酯 | 79-20-9
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-98 °C (lit.)
-
沸点:57-58 °C (lit.)
-
密度:0.934 g/mL at 25 °C
-
蒸气密度:2.55 (vs air)
-
闪点:3.2 °F
-
溶解度:250克/升
-
最大波长(λmax):λ: 255 nm Amax: 1.0λ: 275 nm Amax: 0.1λ: 300 nm Amax: 0.01
-
介电常数:7.3(20℃)
-
暴露限值:TLV-TWA 200 ppm (~610 mg/m3) (ACGIH, MSHA, and OSHA); TLV-STEL 250 ppm (~760 mg/m3) (ACGIH); IDLH 10,000 ppm (NIOSH).
-
LogP:0.18 at 20℃
-
物理描述:Methyl acetate appears as a clear colorless liquid with a fragrant odor. Moderately toxic. Flash point 14°F. Vapors heavier than air.
-
颜色/状态:Colorless, volatile liquid
-
气味:Pleasant odor
-
味道:FLEETING, FRUITY TASTE
-
蒸汽密度:2.8 (NTP, 1992) (Relative to Air)
-
蒸汽压力:216.2 mm Hg at 25 °C
-
亨利常数:1.15e-04 atm-m3/mole
-
大气OH速率常数:3.41e-13 cm3/molecule*sec
-
稳定性/保质期:
-
易挥发、易燃烧、易水解。蒸气与空气形成爆炸性混合物,爆炸极限为4.1%~14.0%(体积),低毒,高浓度时有麻醉作用。干燥时对金属无腐蚀性,但不宜使用铜制容器,因乙酸甲酯容易水解产生的游离乙酸对铜有腐蚀性。回收或久贮的乙酸甲酯在使用前应检查其酸度。接触其蒸气时,应佩戴自吸过滤式防毒面具(半面罩),操作人员需穿防静电工作服,并戴橡胶手套,工作现场严禁吸烟。注意个人清洁卫生。
-
化学性质:乙酸甲酯容易水解,在常温下与水长时间接触也会水解生成乙酸而呈酸性。高温加热时分解成乙醛和甲醛,进一步可分解为甲烷、一氧化碳和氢。卤素,尤其是碘,对分解有促进作用。在镍存在下,150℃以下不发生分解,超过150℃则分解成甲烷、一氧化碳和水。使用铜、银、钼等金属或其氧化物作为催化剂并与空气一同加热时,分解成甲醛与乙酸。紫外线照射可使乙酸甲酯分解为甲醇、丙酮、联乙酰、乙烷、甲烷、氢、一氧化碳和二氧化碳等。在光作用下与氯反应生成氯代乙酸甲酯。在甲醇钠存在下,于57~80℃自行缩合生成乙酰乙酸甲酯。
-
乙酸甲酯能与某些盐类如三氟化硼、三氯化铝、三氯化铁和氯化镍形成复合物,并且也能与氯化钙形成结晶性复合物,因此氯化钙不宜用作乙酸甲酯的干燥剂。
-
乙酸甲酯是纤维素酯、纤维素醚、尿素树脂、蜜胺树脂、乙烯基树脂及酚醛树脂等优良溶剂,但不能溶解虫胶和香豆酮树脂。它能溶解氯化铜、碘化镉、氯化汞、氯化铁和氯化钴等,但不溶解氯化钾、氯化钠和硫酸钠等。常温下在水中可溶解24%,而水在乙酸甲酯中仅能溶解8%。
-
稳定性:稳定
-
禁配物:强氧化剂、碱类及各类酸类
-
聚合危害:不聚合
-
-
自燃温度:850 °F (454 °C)
-
分解:Decomposes in heat; on contact with air, bases, strong oxidizers, UV-light, casuing fire and explosion hazard.
-
粘度:0.364 mPa.s at 25 °C
-
燃烧热:5,150 cal/g = 9,260 BTU/lb = 215X10+5 J/kg
-
汽化热:32.29 kJ/mol at 25 °C
-
表面张力:24.73 mN/m at 25 °C
-
电离电位:10.27 eV
-
气味阈值:WATER: 3.0 MG/L; AIR: 4.6 UL/L; ODOR SAFETY CLASS B; B= 50-90% OF DISTRACTED PERSONS PERCEIVE WARNING OF TLV
-
折光率:Index of refraction: 1.3614 at 20 °C/D
-
保留指数:517.2;506;511;511;521.3;516.7;501.15;511.5;513.8;515.7;520.6;521;521.3;526.2;505;505;505;505;509;560;525.9;509;509;509;505;506;510;512;513;513;509;507;510;511;512;509;513;519.3;515;509;511;556.4;512;510;507;512;513;508;508;513;519;509;513;524
计算性质
-
辛醇/水分配系数(LogP):0.2
-
重原子数:5
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
ADMET
安全信息
-
职业暴露等级:A
-
职业暴露限值:TWA: 200 ppm (610 mg/m3), STEL: 250 ppm (760 mg/m3)
-
TSCA:Yes
-
危险等级:3
-
立即威胁生命和健康浓度:3,100 ppm [10% LEL]
-
危险品标志:F
-
安全说明:S16,S26,S29,S33
-
危险类别码:R66,R36,R67,R11
-
WGK Germany:1
-
海关编码:2915 39 00
-
危险品运输编号:UN 1231 3/PG 2
-
危险类别:3
-
RTECS号:AI9100000
-
包装等级:II
-
危险标志:GHS02,GHS07
-
危险性描述:H225,H319,H336
-
危险性防范说明:P210,P280,P304 + P340 + P312,P305 + P351 + P338,P337 + P313,P403 + P235
-
储存条件:储存注意事项: - 储存于阴凉、通风的库房。 - 远离火种、热源,库温不宜超过37℃。 - 保持容器密封。 - 应与氧化剂、酸类、碱类分开存放,切忌混储。 - 采用防爆型照明和通风设施。 - 禁止使用易产生火花的机械设备和工具。 - 储区应备有泄漏应急处理设备和合适的收容材料。
制备方法与用途
乙酸甲酯(化学式:CH3COOCH3),分子量74.08。无色透明液体,有芳香气味且略带苦味。易燃、易挥发,并在常温下可溶于乙醇、乙醚、水、丙酮、苯和氯仿等有机溶剂中,在20℃时水中的溶解度达31.9g/100ml。天然存在于葡萄和香蕉中。
- 熔点:-98.1°C
- 沸点:57°C (101.3325 kPa)
- 相对密度:0.9330
- 折光率:1.3595
乙酸甲酯的爆炸极限为3.1%~16%,空气中最高允许浓度为200ppm。蒸气有麻醉性,吸入有毒。
含量分析按气相色谱法(GT-10-4)中非极性柱方法测定含量。
毒性乙酸甲酯的急性毒性数据如下:
- 口服LD50 > 5g/kg (大鼠)
- LD50: 2900mg/kg (小鼠,经口)
- 皮试LD50 > 5g/kg (兔子)
作为香料可安全用于食品(FDA, §172.515, 2000),FEMA认定为GRAS(一般认为安全),并经FDA批准食用。欧洲理事会也将乙酸甲酯列入可加入食品中而对人体健康无害的人造食用香料表中,并规定其ADI值为1mg/kg。
使用限量- FEMA (mg/kg):软饮料28;冷饮29;糖果11;焙烤食品14;明胶与布丁0.10;含醇饮料0.20。
乙酸甲酯用于配制香精时,各香料成分不得超过在GB 2760中的最大允许使用量和最大允许残留量。
危险性乙酸甲酯属低毒物质。主要经呼吸道、消化道进入人体,皮肤的吸收量较少。体内可水解产生甲醇,进而生成甲醛和甲酸,其含量与接触剂量成正比。临床表现为黏膜刺激症状、神经系统损害、眼部损害、呼吸系统损伤以及代谢性酸中毒。
化学性质乙酸甲酯又称醋酸甲酯,无色透明液体,有芳香气味且略带苦味。易燃、易挥发,并在常温下可溶于乙醇、乙醚、水、丙酮、苯和氯仿等有机溶剂中,在水中溶解度为31.9g/100ml(20℃)。天然存在于葡萄和香蕉中。
爆炸极限:3.1%~16%
用途 生产方法上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 乙酸乙酯 ethyl acetate 141-78-6 C4H8O2 88.1063 乙酸乙烯酯 vinyl acetate 108-05-4 C4H6O2 86.0904 乙酸酐 acetic anhydride 108-24-7 C4H6O3 102.09 乙酸氯甲酯 Chloromethyl acetate 625-56-9 C3H5ClO2 108.525 丙酸甲酯 Methyl propionate 554-12-1 C4H8O2 88.1063 溶剂黄146 acetic acid 64-19-7 C2H4O2 60.0526 丙烯酸甲酯(MA) acrylic acid methyl ester 96-33-3 C4H6O2 86.0904 氯乙酸甲酯 methyl chloroacetate 96-34-4 C3H5ClO2 108.525 氟乙酸甲酯 methyl fluoroacetate 453-18-9 C3H5FO2 92.0699 溴乙酸甲酯 bromoacetic acid methyl ester 96-32-2 C3H5BrO2 152.975 丙炔酸甲酯 propynoic acid methyl ester 922-67-8 C4H4O2 84.0746 —— methyl 2-iodoacetate 5199-50-8 C3H5IO2 199.976 巯基乙酸甲酯 Methyl thioglycolate 2365-48-2 C3H6O2S 106.145 β-丙内酯 β-Propiolactone 57-57-8 C3H4O2 72.0636 醋酸异丙酯 Isopropyl acetate 108-21-4 C5H10O2 102.133 乙酸丙酯 1-Propyl acetate 109-60-4 C5H10O2 102.133 乙酸烯丙酯 Allyl acetate 591-87-7 C5H8O2 100.117 2-氯乙酸乙酯 2-chloro-1-acetoxyethane 542-58-5 C4H7ClO2 122.551 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 乙酸乙酯 ethyl acetate 141-78-6 C4H8O2 88.1063 甲乙酐 Acetic formic anhydride 2258-42-6 C3H4O3 88.063 乙酸乙烯酯 vinyl acetate 108-05-4 C4H6O2 86.0904 乙酸酐 acetic anhydride 108-24-7 C4H6O3 102.09 乙酸氯甲酯 Chloromethyl acetate 625-56-9 C3H5ClO2 108.525 丙酸甲酯 Methyl propionate 554-12-1 C4H8O2 88.1063 溶剂黄146 acetic acid 64-19-7 C2H4O2 60.0526 丙烯酸甲酯(MA) acrylic acid methyl ester 96-33-3 C4H6O2 86.0904 溴乙酸甲酯 bromoacetic acid methyl ester 96-32-2 C3H5BrO2 152.975 氯乙酸甲酯 methyl chloroacetate 96-34-4 C3H5ClO2 108.525 醋酸异丙酯 Isopropyl acetate 108-21-4 C5H10O2 102.133 乙酸丙酯 1-Propyl acetate 109-60-4 C5H10O2 102.133 乙酸烯丙酯 Allyl acetate 591-87-7 C5H8O2 100.117 2-氯乙酸乙酯 2-chloro-1-acetoxyethane 542-58-5 C4H7ClO2 122.551 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Manufacture of ketenes摘要:公开号:US02175811A1
-
作为产物:参考文献:名称:用于(反式)酯化的酰胺/两性两性离子催化剂:在生物柴油合成中的应用摘要:已经开发了一类基于酰胺阴离子/亚胺阳离子电荷对的两性离子有机催化剂。两性离子易于通过使氮丙啶与氨基吡啶反应来制备。它们催化地适用于酯交换和脱水酯化。机理研究表明,酰胺阴离子和亚胺阳离子在活化反应伙伴方面具有协同作用,亚胺阳离子部分通过非经典氢键与羰基底物相互作用。该反应可在温和条件下用于大规模合成生物柴油。DOI:10.1021/acscatal.9b01959
-
作为试剂:参考文献:名称:Observations and Model Simulations of Carbon Monoxide Emission Factors from a California Highway摘要:A series of twelve intensively monitored 1-hr CO dispersion studies were conducted near Davis, CA, in winter 1996. The experimental equipment included twelve CO sampling ports at elevations up to 50 m, three sonic anemometers, a tethersonde station, aircraft measurements of wind and temperature profile aloft, and a variety of conventional meteorological equipment. The study was designed to explore the role of vehicular exhaust buoyancy during worst-case meteorological conditions, such as low winds oriented in near-parallel alignment with the road during a surface-based nocturnal inversion. From the study, field estimates of the CO emission factor (EF) from a California vehicle fleet were computed using two different methods. The analysis suggests that the CT-EMFAC/EMFAC (EMission FACtor) models currently used to conduct federal conformity modeling significantly overpredict CO emissions for high-speed, free-flowing traffic on California highways.DOI:10.1080/10473289.2001.10464248
文献信息
-
Cyclobutanonesvia the (1-Oxycyclopropyl)methanol Route作者:Ernest Wenkert、Norman F. Golob、Robert P. Hatch、David Wenkert、Roberto PellicciariDOI:10.1002/hlca.19770600102日期:1977.1.261-oxycyclopropyl structure, prapared mostly from α-alkoxy-α,β-unsaturated ketnnes and esters by way of reductinn and Simmons-Smith reaction of the resultant α-alkoxyallyl alcohols, are shown to rearrange into cyclobutanones on acid treatment (cf. Scheme 1).多种1-oxycyclopropyl结构的醇的,从α -烷氧基- α,通过reductinn和的方式β不饱和ketnnes和酯制备大多西蒙斯-史密斯所得α-alkoxyallyl醇的反应中,被示出为重新排列为上cyclobutanones酸处理(参见方案1)。
-
[EN] SUBSTITUTED BENZYLAMINE COMPOUNDS, THEIR USE IN MEDICINE, AND IN PARTICULAR THE TREATMENT OF HEPATITIS C VIRUS (HCV) INFECTION<br/>[FR] COMPOSÉS DE BENZYLAMINE SUBSTITUÉS, LEUR UTILISATION EN MÉDECINE, EN PARTICULIER DANS LE TRAITEMENT D'UNE INFECTION PAR LE VIRUS DE L'HÉPATITE C (VHC)申请人:ASTEX THERAPEUTICS LTD公开号:WO2013064538A1公开(公告)日:2013-05-10The invention provides compounds of the formula (I): or a salt, N-oxide or tautomer thereof, wherein A is CH, CF or nitrogen; E is CH, CF or nitrogen; and R0 is hydrogen or C1-2 alkyl; R1a is selected from CONH2; CO2H; an optionally substituted acyclic C1-8 hydrocarbon group; and an optionally substituted monocyclic carbocyclic or heterocyclic group of 3 to 7 ring members, of which 0, 1, 2, 3 or 4 are heteroatom ring members selected from O, N and S; R2 is selected from hydrogen and a group R2a; R2a is selected from an optionally substituted acyclic d-8 hydrocarbon group; an optionally substituted monocyclic carbocyclic or heterocyclic group of 3 to 7 ring members, of which 0, 1 or 2 ring members are heteroatom ring members selected from O, N and S; and an optionally substituted bicyclic heterocyclic group of 9 or 10 ring members, of which 1 or 2 ring members are nitrogen atoms; wherein at least one of R1 and R2 is other than hydrogen; R3 is an optionally substituted 3- to 10-membered monocyclic or bicyclic carbocyclic or heterocyclic ring containing 0, 1, 2 or 3 heteroatom ring members selected from N, O and S; R4a is selected from halogen; cyano; C1-4 alkyl optionally substituted with one or more fluorine atoms; C1-4 alkoxy optionally substituted with one or more fluorine atoms; hydroxy-C1-4 alkyl; and C1-2 alkoxy-C1-4 alkyl; R5 is selected from hydrogen and a substituent R5a; and R5a is selected from C1-2 alkyl optionally substituted with one or more fluorine atoms; C1-3 alkoxy optionally substituted with one or more fluorine atoms; halogen; cyclopropyl; cyano; and amino, The compounds have activity against hepatitis C virus and can be used in the prevention or treatment of hepatitis C viral infections.该发明提供了以下式(I)的化合物,或其盐、N-氧化物或互变异构体,其中A为CH、CF或氮;E为CH、CF或氮;R0为氢或C1-2烷基;R1a选自CONH2;CO2H;一个可选择取代的非环状C1-8碳氢化合物基团;以及一个可选择取代的含有3至7个环成员的单环碳环或杂环基团,其中0、1、2、3或4个是从O、N和S中选择的杂原子环成员;R2选自氢和一个基团R2a;R2a选自一个可选择取代的非环状d-8碳氢化合物基团;一个可选择取代的含有3至7个环成员的单环碳环或杂环基团,其中0、1或2个环成员是从O、N和S中选择的杂原子环成员;以及一个可选择取代的含有9或10个环成员的双环杂环基团,其中1或2个环成员是氮原子;其中R1和R2中至少一个不是氢;R3选自一个可选择取代的含有0、1、2或3个从N、O和S中选择的杂原子环成员的3至10个成员的单环或双环碳环或杂环环;R4a选自卤素;氰基;C1-4烷基,可选择取代一个或多个氟原子;C1-4烷氧基,可选择取代一个或多个氟原子;羟基-C1-4烷基;和C1-2烷氧基-C1-4烷基;R5选自氢和一个取代基R5a;R5a选自C1-2烷基,可选择取代一个或多个氟原子;C1-3烷氧基,可选择取代一个或多个氟原子;卤素;环丙基;氰基;和氨基。这些化合物对丙型肝炎病毒具有活性,并可用于预防或治疗丙型肝炎病毒感染。
-
[EN] COMPOUNDS INHIBITING LEUCINE-RICH REPEAT KINASE ENZYME ACTIVITY<br/>[FR] COMPOSÉS INHIBANT L'ACTIVITÉ ENZYMATIQUE DE LA KINASE À MOTIFS RÉPÉTÉS RICHES EN LEUCINE申请人:MERCK SHARP & DOHME公开号:WO2014137723A1公开(公告)日:2014-09-12The present invention is directed to indazole compounds which are potent inhibitors of LRRK2 kinase and useful in the treatment or prevention of diseases in which the LRRK2 kinase is involved, such as Parkinson's Disease. The invention is also directed to pharmaceutical compositions comprising these compounds and the use of these compounds and compositions in the prevention or treatment of such diseases in which LRRK-2 kinase is involved.
-
[EN] COMPOUNDS INHIBITING LEUCINE-RICH REPEAT KINASE ENZYME ACTIVITY<br/>[FR] COMPOSÉS INHIBANT L'ACTIVITÉ ENZYMATIQUE DE LA KINASE À SÉQUENCE RÉPÉTÉE RICHE EN LEUCINE申请人:MERCK SHARP & DOHME公开号:WO2014134774A1公开(公告)日:2014-09-12Disclosed are indazole compounds which are potent inhibitors of LRRK2 kinase and useful in the treatment or prevention of diseases in which LRRK2 kinase is involved. Also disclosed are pharmaceutical compositions in the prevention or treatment of such diseases in which LRRK2 kinase is involved.揭示了一种indazole化合物,它们是LRRK2激酶的有效抑制剂,并且在涉及LRRK2激酶的疾病的治疗或预防中有用。还揭示了在涉及LRRK2激酶的这类疾病的预防或治疗中使用的药物组合物。
-
Asymmetric Synthesis of Natural <i>cis</i>-Dihydroarenediols Using Tetrahydroxynaphthalene Reductase and Its Biosynthetic Implications作者:Nirmal Saha、Michael Müller、Syed Masood HusainDOI:10.1021/acs.orglett.9b00500日期:2019.4.5Asymmetric reduction of hydroxynaphthoquinones to secondary metabolites, (3S,4R)-3,4,8- and (2S,4R)-2,4,8-trihydroxy-1-tetralone, a putative biosynthetic diketo intermediate and a probable natural analogue, (3S,4R)-7-acetyl-3,4,8-trihydroxy-6-methyl-3,4-dihydronaphthalene-1(2H)-one, using NADPH-dependent tetrahydroxynaphthalene reductase (T4HNR) of Magnaporthe grisea is described. This work implies
表征谱图
-
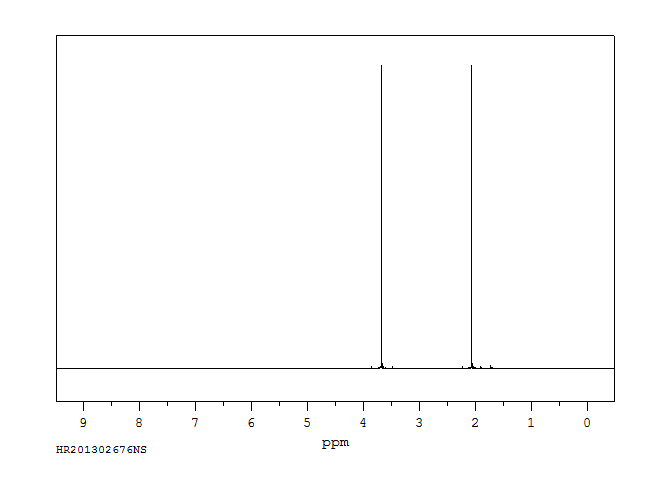
氢谱1HNMR
-
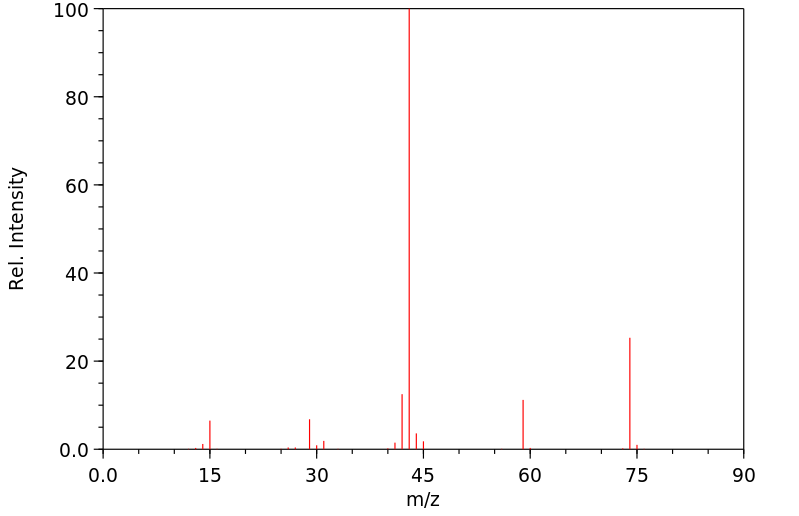
质谱MS
-
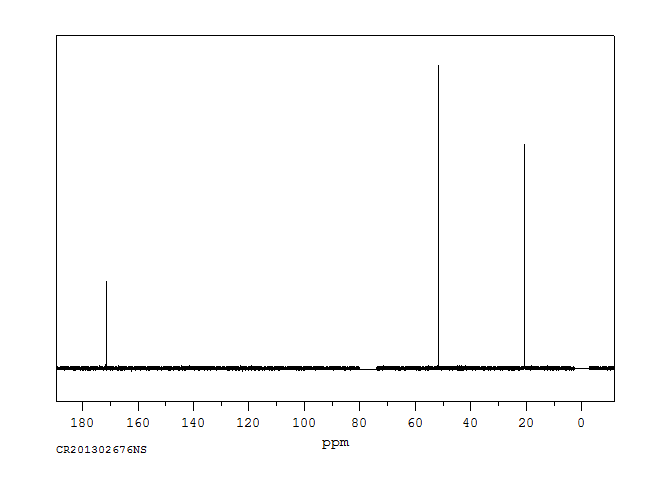
碳谱13CNMR
-
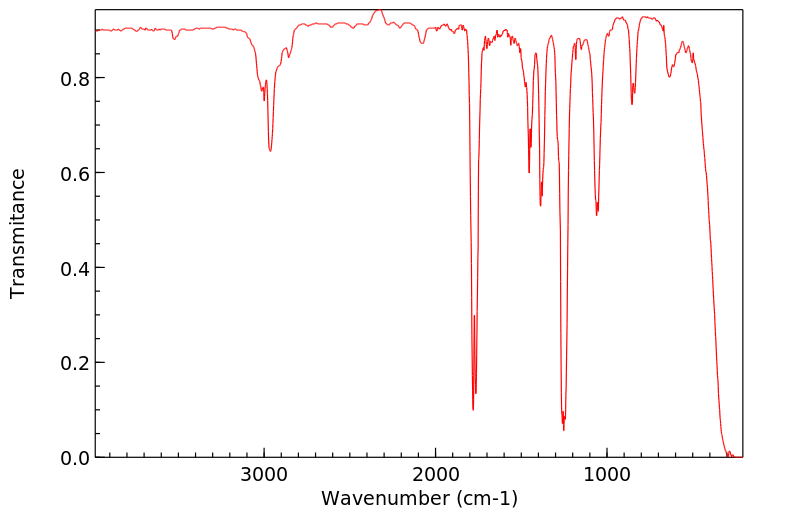
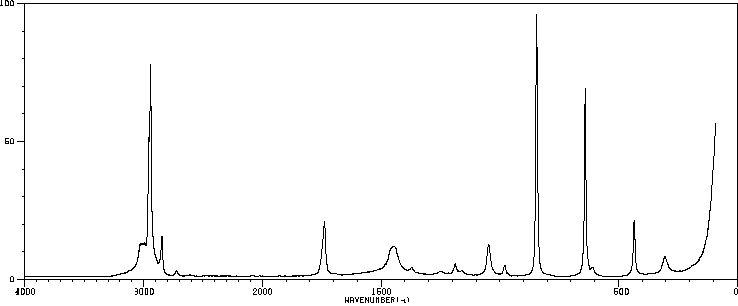
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息