十二烷醇 | 112-53-8
中文名称
十二烷醇
中文别名
月桂醇;C12醇;正十二烷醇;1-十二烷醇;十二醇;十二碳醇;正十二醇;12醇;1-十二醇
英文名称
1-Dodecanol
英文别名
1-dodecyl alcohol;dodecanol;lauryl alcohol;dodecan-1-ol;n-dodecanol;dodecyl alcohol;1‐dodecanol;n-dodecyl alcohol
CAS
112-53-8;27342-88-7
化学式
C12H26O
mdl
MFCD00004753
分子量
186.338
InChiKey
LQZZUXJYWNFBMV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:22-26 °C(lit.)
-
沸点:260-262 °C(lit.)
-
密度:0.833 g/mL at 25 °C(lit.)
-
蒸气密度:7.4 (vs air)
-
闪点:>230 °F
-
溶解度:23°C时微溶于水,溶解度为1g/L
-
介电常数:6.5(24℃)
-
LogP:5.4 at 23℃
-
物理描述:Dodecanol is a colorless thick liquid with a sweet odor. Floats on water. Freezing point is 75°F. (USCG, 1999)
-
颜色/状态:COLORLESS LIQ AT ROOM TEMP; CRYSTALLINE OR FLAKES BELOW 20 °C
-
气味:CHARACTERISTIC FATTY ODOR; UNPLEASANT AT HIGH CONCN BUT DELICATE & FLORAL ON DILUTION
-
味道:FATTY, WAXY FLAVOR
-
蒸汽密度:6.43 (AIR= 1)
-
蒸汽压力:8.48X10-4 mm Hg at 25 °C
-
亨利常数:2.22e-05 atm-m3/mole
-
自燃温度:527 °F (275 °C)
-
分解:When heated to decomposition it emits acrid smoke and fumes.
-
粘度:18.8 mPa-s at 20 °C
-
燃烧热:-7.3380X10+9 J/kmol
-
汽化热:90.8 kJ/mol at 25 °C
-
表面张力:0.029493 N/m at melting point
-
气味阈值:The air odor threshold for dodecyl alcohol (isomer not specified) is reported as 7.1 ppb.
-
折光率:Index of refraction: 0.8309 at 24 °C/4 °C (liq)
-
保留指数:1457.1;1474;1468.9;1456;1462;1460;1456.5;1456.8;1442;1443;1455.1;1463.1;1456.6;1457;1459;1461;1472;1477;1442;1448;1449;1450;1451;1451;1458;1432;1463;1465;1458;1471;1468;1468;1472;1472;1474;1474;1470;1472;1457;1474;1451;1468;1468;1468;1465;1463.1;1457;1460;1461;1468;1462;1458;1462;1488;1462.2;1460
计算性质
-
辛醇/水分配系数(LogP):5.1
-
重原子数:13
-
可旋转键数:10
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
ADMET
代谢
青蛙肝脏微粒体催化了1-十二醇的羟基化,生成了相应的omega-和(omega-1)-羟基衍生物。1-十二醇的羟基化速率远低于月桂酸的羟基化速率。羟基化活性需要NADPH和O2。NADH对羟基化没有影响。在CO:O2比例为4.0时,CO使羟基化系统活性抑制了49%。CO对omega-羟基十二醇的形成抑制更为明显,比对(omega-1)-羟基十二醇的形成抑制更为显著,这表明不止一种细胞色素P-450参与了1-十二醇的羟基化,并且CO对催化omega-羟基化的P-450有更高的亲和力。在1-十二醇与青蛙肝脏微粒体一起孵化时,月桂酸的形成表明微粒体中也存在脂肪醇氧化系统。NAD+是1-十二醇氧化的最有效辅因子,而NADP+对此几乎没有影响。吡唑(一种醇脱氢酶抑制剂)对氧化有轻微的抑制作用,而叠氮化钠(一种过氧化氢酶抑制剂)没有影响。
Frog liver microsomes catalyzed the hydroxylation of 1-dodecanol into the corresponding omega- and (omega-1)-hydroxy derivatives. The hydroxylation rate for 1-dodecanol was much lower than that for lauric acid. Both NADPH and O2 were required for hydroxylation activity. NADH had no effect on the hydroxylation. The hydroxylating system was inhibited 49% by CO at a CO:O2 ratio of 4.0. The formation of omega-hydroxydodecanol was more sharply inhibited by CO than was the formation of (omega-1)-hydroxydodecanol, implying that more than one cytochrome P-450 was involved in the hydroxylation of 1-dodecanol and that CO has a higher affinity for the P-450 catalyzing the omega-hydroxylation. The formation of laurate during the incubation of 1-dodecanol with frog liver microsomes suggests that a fatty alcohol oxidation system is also present in the microsomes. NAD+ was the most effective cofactor for the oxidation of 1-dodecanol and NADP+ had a little effect. Pyrazole (an inhibitor of alcohol dehydrogenase) had a slight inhibitory effect on the oxidation and sodium azide (an inhibitor of catalase) had no effect.
来源:Hazardous Substances Data Bank (HSDB)
代谢
蒙古沙鼠(Meriones unguiculatus)的肝微粒体催化了各种饱和脂肪酸(C8-C18)、醇(C12和C16)和烃(C12)的羟基化,生成相应的ω-和(ω-1)-羟基衍生物。在饱和脂肪酸中,月桂酸被羟基化的效果最显著,羟基化底物的活性顺序是C12 > C14 > C13 > C16 > C10 > C18 > C8。沙鼠肝微粒体中月桂酸羟基化的特定活性(5.99 nmol/mg微粒体蛋白/min)高于其他物种观察到的活性。1-十二醇也被沙鼠肝微粒体非常有效地羟基化(4.58 nmol/mg微粒体蛋白/min),但一般来说,脂肪酸醇的羟基化速率远低于相应酸的羟基化速率。
The liver microsomes of the Mongolian gerbil Meriones unguiculatus catalyzed the hydroxylation of various saturated fatty acids (C8-C18), alcohols (C12 and C16) and hydrocarbon (C12) to the corresponding omega- and (omega-1)-hydroxy derivatives. Lauric acid was hydroxylated most effectively among saturated fatty acids and the order of activity as hydroxylation substrates was C12 greater than C14 greater than C13 greater than C16 greater than C10 greater than C18 greater than C8. The specific activity of laurate hydroxylation (5.99 nmol/mg microsomal protein/min) in gerbil liver microsomes was higher than that observed in other species. 1-Dodecanol was also hydroxylated very effectively (4.58 nmol/mg microsomal protein/min) by gerbil liver microsomes, but in general the hydroxylation rates for fatty alcohols were much lower than those for the corresponding acids.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
识别和使用:1-十二醇主要用于作为n-十二烷基硫酸盐的化学中间体和酒精硫酸盐表面活性剂的泡沫稳定剂。它还用于合成洗涤剂、润滑剂添加剂、药品、橡胶、纺织品、香水和作为调味剂。该物质实际上是无毒的,并且在美国和欧盟都被允许作为食品添加剂(公认安全GRAS)。它在美国注册为杀虫剂使用,但批准的杀虫剂用途可能会定期更改,因此必须咨询联邦、州和地方当局以获取当前批准的用途。
人类暴露和毒性:25名志愿者与含有4% 1-十二醇的石油脂接触48小时并未引起刺激,但当5到10名志愿者的刮伤皮肤与含有25% 1-十二醇的矿物油进行开放接触,每天一次,连续3天时,出现了明显的皮肤刺激。在25名志愿者中,4%的1-十二醇浓度在石油脂中并未引起皮肤致敏。
动物研究:向10只大鼠中吸入0.2毫升的1-十二醇导致其中9只死亡。死亡原因是肺水肿,而不是C3到C10醇类引起的心脏停搏或呼吸衰竭。肺部呈深红色;七只大鼠在7至30分钟内死亡,两只大鼠在给药后5小时或更长时间死亡。在大鼠中进行测试时,1-十二醇在0、100、500、2000 mg/kg/天的浓度下,在配对前14天内并未引起发育或生殖毒性。在给予的剂量下,1-十二醇对胎鼠体重、体重增加、食物消耗和食物效率的亲代没有影响。在进行致癌性测试时,1-十二醇在每周三次,持续60周的应用下,对之前接受过二甲基苯[a]蒽启动剂量的小鼠皮肤表现出微弱的肿瘤促进作用。在另一项研究中,6-8周大的小鼠,每周三次,连续八周腹腔注射1-十二醇溶于0.1毫升的三辛酸甘油酯(高剂量组30只动物12.0 g/kg,低剂量组28只动物2.4 g/kg)。治疗39周和49周后,30只小鼠中有2只出现了乳头状瘤。在高剂量组的15只雌性小鼠中有2只观察到肺肿瘤,在低剂量组的15只雄性和13只雌性小鼠中各有2只和3只观察到肺肿瘤。1-十二醇在有无代谢活化的情况下对沙门氏菌属伤寒杆菌的Ames试验,或对大肠杆菌的无代谢活化情况下均不具有诱变性。然而,它在14小时暴露后减少了蚕豆细胞的分裂活性,并引起了染色体的结构变化和有丝分裂装置。
生态毒性:尽管1-十二醇对水生生物表现出非极性中枢神经系统抑制剂毒性,大约为1 mg/L,但该物质易于降解,在生产过程中或在自由醇的广泛使用中释放,并不会引起环境问题。
IDENTIFICATION AND USE: 1-Dodecanol is used principally as chemical intermediate for salts of n-dodecyl sulfate and foam stabilizer for alcohol sulfate surfactants. It is also used in synthetic detergents, lube additives, pharmaceuticals, rubber, textiles, perfumes, and as a flavoring agent. The substance is practically non-toxic, and is a permitted food additive (GRAS) in both the U.S. and the EU. It is registered for pesticide use in the U.S. but approved pesticide uses may change periodically and so federal, state and local authorities must be consulted for currently approved uses. HUMAN EXPOSURE AND TOXICITY: Contact for 48 hours with 4% 1-dodecanol in petrolatum was not irritating to 25 human volunteers, but marked skin irritation was noted when 25% 1-dodecanol in mineral oil was given in open contact with scarified skin of 5 to 10 volunteers once a day for 3 days. There was no skin sensitization in 25 human volunteers at a concentration of 4% in petrolatum. ANIMAL STUDIES: Aspiration of 0.2 mL of 1-dodecanol produced death among 9 out of 10 rats. The deaths were caused by pulmonary edema rather than cardiac arrest or respiratory failure as with the C3 to C10 alcohols. The lungs were dark red; seven rats died within 7 to 30 min, and two rats died 5 hr or longer after dosing. 1-Dodecanol did not cause developmental or reproductive toxicities when tested in rats at concentrations of 0, 100, 500, 2000 mg/kg /day for 14 days prior to mating. 1-Dodecanol, in the doses administered, had no effect on fetal weight, weight gain, food consumption, and food efficiency in the parental generation. When tested for carcinogenicity, 1-dodecanol showed weak tumor-promoting activity when applied three times a week for 60 weeks to the skin of mice that had previously received an initiating dose of dimethylbenz[a]anthracene. In another study, in 6-8 week old mice, ip injections of dodecanol in 0.1 mL tricaprylin (12.0 g/kg to 30 animals in high dose group, and 2.4 g/kg to 28 animals in low dose group) was administered 3 times weekly for eight weeks. Papillomas developed in 2 of 30 mice after 39 and 49 weeks of treatment. Lung tumors were observed in 2/15 female mice in the high dose group, and in 2/15 males and 3/13 females in the low dose group. 1-Dodecanol was not mutagenic to Salmonella typhimurium in the Ames assay with and without metabolic activation, or to Escherichia coli without metabolic activation. However, it diminished cell mitotic activity and caused structural changes to chromosomes and the mitotic apparatus in Vicia faba after 14 hours exposure. ECOTOXICITY: Although Dodecanol exhibits non-polar CNS depressant toxicity to aquatic organisms of about 1 mg/L, the substance is readily degradable and releases during production, or through diffuse uses of the free alcohol do not give rise to environmental concerns.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
这种物质可以通过吸入、皮肤接触和摄入被身体吸收。
The substance can be absorbed into the body by inhalation, through the skin and by ingestion.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
咳嗽。喉咙痛。
Cough. Sore throat.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
Redness.
Redness.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
红斑。疼痛。
Redness. Pain.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
吸收、分配和排泄
在接触小鼠皮肤24小时后,大约95%的100微升0.5%的月桂醇三乙基柠檬酸盐剂量留在皮肤上。一小部分,0.1%,在粪便和尿液中回收,0.13%从体内回收,2.61%通过空气排出。这些数据表明皮肤吸收量较低。
After 24-hr covered contact with the skin of mice, about 95% of a 100-uL dose of 0.5% 1-dodecanol in triethyl citrate remained on the skin. A small proportion, 0.1%, was recovered in the feces and urine, 0.13% was recovered from the body, and 2.61% was excreted in the air. These data indicate a low amount of dermal uptake.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
TSCA:Yes
-
危险等级:9
-
危险品标志:Xi
-
安全说明:S26,S29,S36,S37/39,S60,S61
-
危险类别码:R36/38,R50/53
-
WGK Germany:1
-
海关编码:2905170000
-
危险品运输编号:UN 3077 9/PG 3
-
危险类别:9
-
RTECS号:JR5775000
-
包装等级:III
-
危险标志:GHS07,GHS09
-
危险性描述:H319,H410
-
危险性防范说明:P305 + P351 + P338
SDS
制备方法与用途
十二醇
简介
十二醇是一种有刺激性气味的油状液体或固体,具有可燃性和低毒性。它不溶于水,但可以溶解在乙醇和乙醚等有机溶剂中。与浓硫酸反应会发生酯化作用,生成产物十二烷基硫酸(硫酸十二烷酯),进一步引入钠离子则形成十二烷基硫酸钠(SDS)。该物质常用于牙膏、肥皂等清洁用品的生产过程中。然而,当它与碱性物质接触时通常不会发生显著反应。
应用作为一种脂肪醇,十二醇是洗涤剂、表面活性剂、塑料增塑剂等精细化工产品的基础原料之一。由其衍生出的产品种类繁多,并广泛应用于化工、石油、冶金、纺织等多个行业领域。近年来对这种物质的需求持续增长。
理化性质十二醇为无色液体,具有微弱且持久的油脂气味,在温度低于20℃时会凝固成固体形态。它不溶于水,但能溶解在乙醚和乙醇中,并具备一定的可燃性与空气形成爆炸混合物的能力。此外,对人体的眼睛和皮肤有轻微刺激作用。
含量分析可以通过总醇量测定法(OT-5)或气相色谱法(GT-10-4)进行含量检测。具体操作中取样量为1.5g,并使用当量因子93.17来进行计算。
毒性十二醇的ADI值为1 mg/kg,而其LD₅₀约为12800 mg/kg(大鼠经口摄入)。
使用限量FEMA对各种食品类型中允许使用的最大用量有明确规定:软饮料2.0 mg/kg;冷饮1.0 mg/kg;糖果2.8 mg/kg;焙烤食品1.7 mg/kg;胶姆糖16~27 mg/kg;糖浆7.0 mg/kg。该物质被美国食品药品监督管理局认定为适度限用。
食品添加剂最大允许使用量及残留标准 化学性质十二醇是一种淡黄色油状液体或固体,在常温下相对稳定。它具有月下香及紫罗兰的香气,适用于制造玫瑰型、紫罗兰型和百合水仙型香精。
生产方法- 高压加氢法:椰子油在铜铬催化剂中连续加氢得到C8-C18混合醇;随后进行常压蒸馏分离C8-C10醇、C12-C14醇及C16-C18醇。
- 酯化加氢法:椰子油与甲醇发生酯交换反应生成月桂酸甲酯和甘油,经催化加氢并蒸馏后可得十二醇。
- 易燃液体
- 中毒性分级为中毒级别
- 急性毒性:
- 大鼠口服LD₅₀: 12800 毫克/ 公斤
- 刺激数据:
- 皮肤刺激:人,75毫克/3天,重度
- 可燃危险特性:
- 遇明火、高温或强氧化剂可燃;燃烧时排放刺激性烟雾
- 储运特性:
- 库房应通风且保持低温干燥状态储存和运输。
- 灭火剂推荐:泡沫、干粉、二氧化碳或砂土覆盖。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 十三烷醇 1-Tridecanol 112-70-9 C13H28O 200.365 1,8-辛二醇 1,8-Octanediol 629-41-4 C8H18O2 146.23 1,12-十二烷二醇 1,12-dodecandiol 5675-51-4 C12H26O2 202.337 十四醇 1-Tetradecanol 112-72-1 C14H30O 214.392 1-十五醇 pentadecanol 629-76-5 C15H32O 228.418 1-十六烷醇 1-Hexadecanol 36653-82-4 C16H34O 242.445 硬脂醇 1-octadecanol 112-92-5 C18H38O 270.499 1-甲氧基十二烷 dodecyl methyl ether 3482-63-1 C13H28O 200.365 8-溴-1-辛醇 8-bromooctanol 50816-19-8 C8H17BrO 209.126 12-溴-1-十二烷醇 12-bromododecanol 3344-77-2 C12H25BrO 265.234 O-十二基羟胺 O-dodecylhydroxylamine 40345-87-7 C12H27NO 201.352 戊醇 pentan-1-ol 71-41-0 C5H12O 88.1497 9-十二炔-1-醇 9-dodecyn-1-ol 71084-08-7 C12H22O 182.306 油醇 oleoyl alcohol 143-28-2 C18H36O 268.483 十二烷基乙烯基醚 1-(vinyloxy)dodecane 765-14-0 C14H28O 212.376 月桂酸 laurate 143-07-7 C12H24O2 200.321 十二烷基甲酸酯 dodecyl formate 28303-42-6 C13H26O2 214.348 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,12-十二烷二醇 1,12-dodecandiol 5675-51-4 C12H26O2 202.337 十三烷醇 1-Tridecanol 112-70-9 C13H28O 200.365 十二烷-1,11-二醇 1,11-dodecane diol 80158-99-2 C12H26O2 202.337 2-十三醇 tridecan-2-ol 1653-31-2 C13H28O 200.365 —— (S)-(+)-tridecan-2-ol 1653-31-2 C13H28O 200.365 1-甲氧基十二烷 dodecyl methyl ether 3482-63-1 C13H28O 200.365 —— hypochlorous acid dodecyl ester 78132-57-7 C12H25ClO 220.783 14-十五炔-1-醇 pentadec-14-yn-1-ol 18202-13-6 C15H28O 224.387 —— dodecanol-(2)H 14848-65-8 C12H26O 187.33 —— dodecyl hydroperoxide 3229-98-9 C12H26O2 202.337 O-十二基羟胺 O-dodecylhydroxylamine 40345-87-7 C12H27NO 201.352 —— 1,10-dihydroxydodecane 39516-27-3 C12H26O2 202.337 2-甲基-1-十二烷醇 2-methyl-1-dodecanol 22663-61-2 C13H28O 200.365 双十二烷基乙醚 dilauryl ether 4542-57-8 C24H50O 354.66 —— n-Butyl-dodecylether 7289-38-5 C16H34O 242.445 —— Heptyl-dodecylaether 17146-24-6 C19H40O 284.526 —— 1,4-dodecanediol 38146-95-1 C12H26O2 202.337 —— 1,6-bis-dodecyloxy-hexane 103399-25-3 C30H62O2 454.821 十二烷基乙烯基醚 1-(vinyloxy)dodecane 765-14-0 C14H28O 212.376 月桂酸 laurate 143-07-7 C12H24O2 200.321 1-(氯甲氧基)十二烷 chloromethyldodecyl ether 13497-61-5 C13H27ClO 234.81 十二烷基甲酸酯 dodecyl formate 28303-42-6 C13H26O2 214.348 十三酸 tridecanoic acid 638-53-9 C13H26O2 214.348 - 1
- 2
- 3
反应信息
-
作为反应物:描述:十二烷醇 在 methoxy(cyclooctadiene)rhodium(I) dimer 、 3-甲氧基苯甲酸 、 丙酮 、 4,5-双二苯基膦-9,9-二甲基氧杂蒽 作用下, 以 甲苯 为溶剂, 反应 24.0h, 以15%的产率得到十一烷参考文献:名称:串联催化:通过氧化脱羟甲基化将醇转化为烯烃摘要:我们报道了一种 Rh 催化剂,用于通过 CC 键断裂从伯醇中获取烯烃,从而导致脱同系反应。这种官能团互变通过 N,N-二甲基丙烯酰胺作为氢气的牺牲受体实现的氧化-脱氢甲酰化进行。具有不同官能度和结构的醇经过氧化脱羟甲基反应得到相应的烯烃。我们的催化剂方案能够实现 (+)-育亨苯酮的两步半合成和原料烯烃的脱同系。DOI:10.1021/jacs.8b06069
-
作为产物:参考文献:名称:Aqueous polar aprotic solvents. Efficient sources of nucleophilic oxygen摘要:DOI:10.1021/jo00156a045
-
作为试剂:描述:参考文献:名称:Kinetic Model for Studying the Effect of Quercetin on Cholesterol Oxidation during Heating摘要:Inhibition of the heat-induced cholesterol oxidation at 150 degrees C by incorporation of quercetin was kinetically studied. Results showed that without quercetin, the cholesterol oxidation products (COPs) concentration increased with increasing heating time. A low amount (0.002%, w/w) of quercetin was effective in inhibiting the formation of COPs during the initial heating period (<= 30 min) at 150 degrees C. However, after prolonged heating (30-120 min), a low antioxiclant activity was observed because of the degradation of quercetin. When using nonlinear regression models for kinetic study of cholesterol oxidation in the absence of quercetin, the epoxidation showed the highest rate constant (h(-1) = 683.1), followed by free radical chain reaction (h(-1) = 453.5), reduction (h(-1) = 290.3), dehydration (h(-1) = 155.5), triol dehydrogenation (h(-1) = 5.35), dehydrogenation (h(-1) = 0.68), thermal degradation (h(-1) = 0.66), and triol formation (h(-1) = 0.38). However, in the presence of quercetin, the reaction rate constants (h(-1)) for epoxidation (551.4), free radical chain reaction (111.7), and thermal degradation (0.28) were reduced greatly. The kinetic model developed in this study can be used to predict the inhibition of COPs by quercetin during the heating of cholesterol.DOI:10.1021/jf052529r
文献信息
-
Additives and products including oligoesters申请人:——公开号:US20030199593A1公开(公告)日:2003-10-23The present invention relates to oligoesters and their use or the creation of additives. Oligoester containing additives and/or oligoesters themselves may be used for formulating pharmaceutical preparations, cosmetics or personal care products such as shampoos and conditioners. These oligoesters are particularly useful for the creation of multi-purpose additives that can impart conditioning, long substantivity and/or UV protection. Individual oligoesters and oligoester mixtures are described.本发明涉及寡酯及其用途或添加剂的制备。含有寡酯的添加剂和/或寡酯本身可用于配制药物制剂、化妆品或个人护理产品,如洗发水和护发素。这些寡酯对于制备能够赋予调理、长效性和/或紫外线保护的多功能添加剂特别有用。描述了单独的寡酯和寡酯混合物。
-
[EN] 3-[(HYDRAZONO)METHYL]-N-(TETRAZOL-5-YL)-BENZAMIDE AND 3-[(HYDRAZONO)METHYL]-N-(1,3,4-OXADIAZOL-2-YL)-BENZAMIDE DERIVATIVES AS HERBICIDES<br/>[FR] DÉRIVÉS DE 3-[(HYDRAZONO))MÉTHYL]-N-(TÉTRAZOL-5-YL)-BENZAMIDE ET DE 3-[(HYDRAZONO)MÉTHYL]-N-(1,3,4-OXADIAZOL-2-YL)-BENZAMIDE UTILISÉS EN TANT QU'HERBICIDES申请人:SYNGENTA CROP PROTECTION AG公开号:WO2021013969A1公开(公告)日:2021-01-28The present invention related to compounds of Formula (I): or an agronomically acceptable salt thereof, wherein Q, R2, R3, R4, R5 and R6 are as described herein. The invention further relates to compositions comprising said compounds, to methods of controlling weeds using said compositions, and to the use of compounds of Formula (I) as a herbicide.本发明涉及以下式(I)的化合物或其农业上可接受的盐,其中Q、R2、R3、R4、R5和R6如本文所述。该发明还涉及包含所述化合物的组合物,使用这些组合物控制杂草的方法,以及将式(I)的化合物用作除草剂的用途。
-
[EN] DIHYDROPYRROLONAPHTYRIDINONE COMPOUNDS AS INHIBITORS OF JAK<br/>[FR] COMPOSÉS DE DIHYDROPYRROLONAPHTYRIDINONE COMME INHIBITEURS DE JAK申请人:TAKEDA PHARMACEUTICAL公开号:WO2010144486A1公开(公告)日:2010-12-16Disclosed are JAK inhibitors of formula (I) where G1, R1, R2, R3, R4, R5, R6, and R7 are defined in the specification. Also disclosed are pharmaceutical compositions, kits and articles of manufacture which contain the compounds, methods and materials for making the compounds, and methods of using the compounds to treat diseases, disorders, and conditions involving the immune system and inflammation, including rheumatoid arthritis, hematological malignancies, epithelial cancers (i.e., carcinomas), and other diseases, disorders or conditions associated with JAK.揭示了式(I)的JAK抑制剂,其中G1、R1、R2、R3、R4、R5、R6和R7在规范中定义。还披露了含有这些化合物的药物组合物、试剂盒和制造物品,制备这些化合物的方法和材料,以及使用这些化合物治疗涉及免疫系统和炎症的疾病、紊乱和症状的方法,包括类风湿关节炎、血液恶性肿瘤、上皮癌(即癌症)和其他与JAK相关的疾病、紊乱或症状。
-
[EN] INSECTICIDAL TRIAZINONE DERIVATIVES<br/>[FR] DÉRIVÉS DE TRIAZINONE INSECTICIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2013079350A1公开(公告)日:2013-06-06Compounds of the formula (I) or (I'), wherein the substituents are as defined in claim 1, are useful as pesticides.式(I)或(I')的化合物,其中取代基如权利要求1所定义的那样,可用作杀虫剂。
-
Probing a sialyltransferase’s recognition domain to prepare α(2,8)-linked oligosialosides and analogs作者:Ping Zhang、Amir J. Zuccolo、Wenling Li、Ruixiang Blake Zheng、Chang-Chun LingDOI:10.1039/b908933k日期:——We report the first observation that a monosialyl residue is the essential structural element recognized by the enzyme CST-II; this has resulted in an attractive route to synthesize a series of α(2,8)-linked oligosialic acids and their thioanalogs in one step.
表征谱图
-
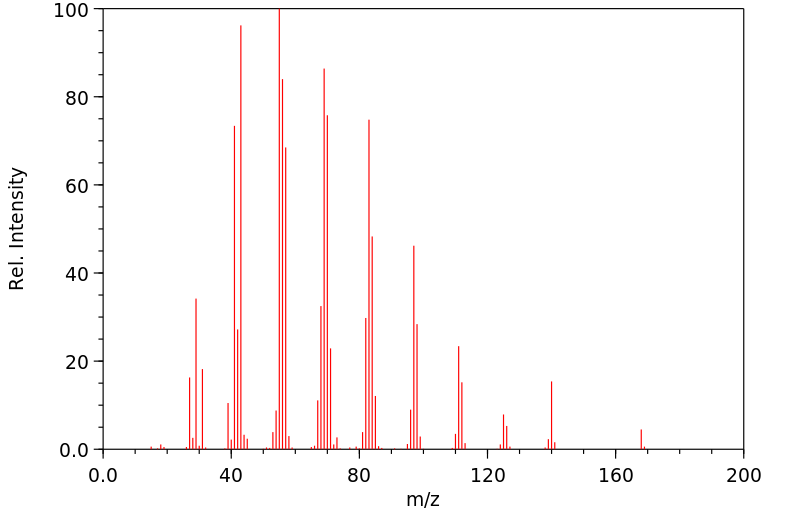
氢谱1HNMR
-
质谱MS
-
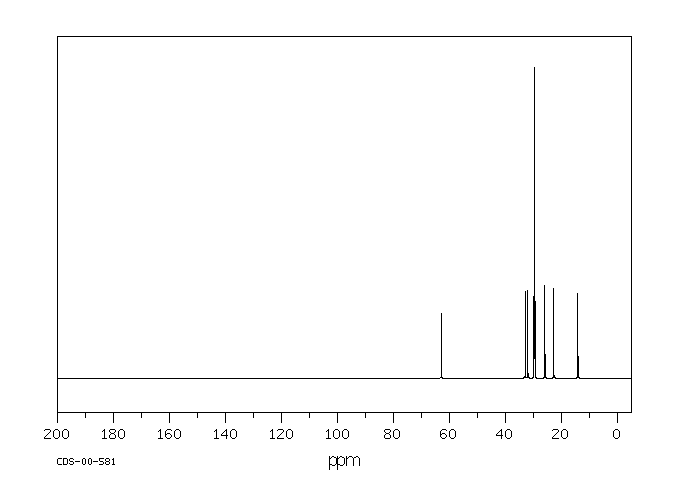
碳谱13CNMR
-
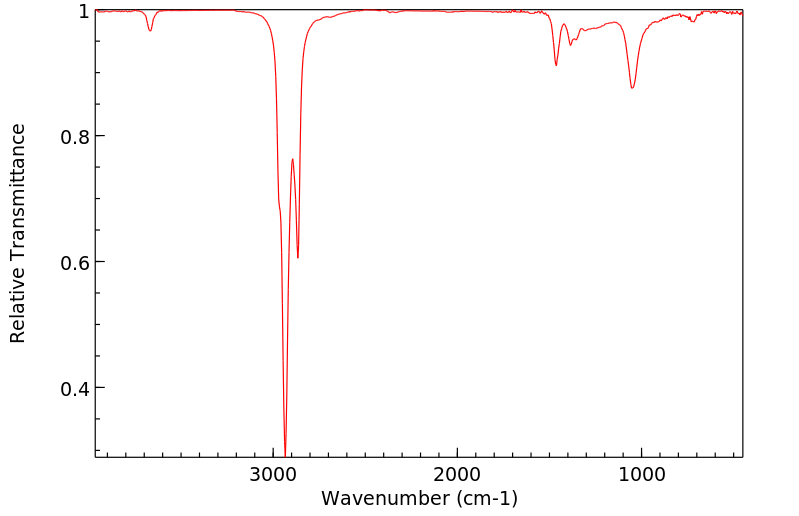
红外IR
-
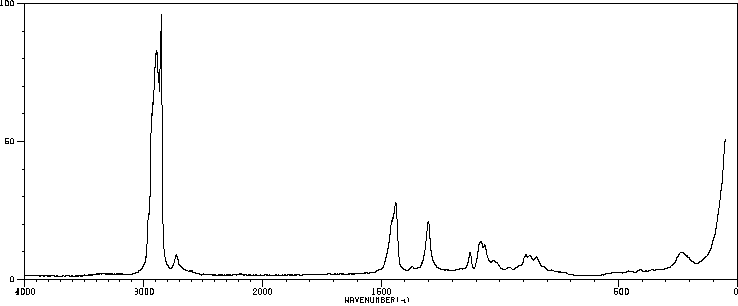
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯