1-(4-乙烯基-苯基)-乙酮 | 10537-63-0
物质功能分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:33 °C
-
沸点:110 °C(Press: 12 Torr)
-
密度:0.995±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2914399090
-
储存条件:存储条件:2-8°C,密封,干燥。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-乙酰苯甲醛 p-formylacetophenone 3457-45-2 C9H8O2 148.161 对乙基苯乙酮 4-ethylacetophenone 937-30-4 C10H12O 148.205 1,4-二乙酰苯 1,4-Diacetylbenzene 1009-61-6 C10H10O2 162.188 1-[4-(2-氨基乙基)苯基]-乙酮 1-[4-(2-Amino-ethyl)-phenyl]-ethanone 31349-78-7 C10H13NO 163.219 4-(2-溴乙基)苯乙酮 1-[4-(2-bromoethyl)phenyl]ethanone 40422-73-9 C10H11BrO 227.101 4-(2-氯乙基)苯乙酮 1-(4-(2-chloroethyl)phenyl)ethan-1-one 69614-95-5 C10H11ClO 182.65 4-乙炔基苯乙酮 4-ethynylacetophenone 42472-69-5 C10H8O 144.173 —— 1-(4-acetylphenyl)ethanol 15519-23-0 C10H12O2 164.204 对羟基苯乙酮 4-Hydroxyacetophenone 99-93-4 C8H8O2 136.15 rac-1-(4-乙烯基苯基)乙醇 1-(4-vinylphenyl)ethanol 17250-77-0 C10H12O 148.205 对氯苯乙酮 para-chloroacetophenone 99-91-2 C8H7ClO 154.596 4-乙烯基苯甲酸 4-Vinylbenzoic acid 1075-49-6 C9H8O2 148.161 4-碘代苯乙酮 4-Iodoacetophenone 13329-40-3 C8H7IO 246.047 4-溴苯乙酮 para-bromoacetophenone 99-90-1 C8H7BrO 199.047 4-乙烯基苯甲酰氯 p-vinylbenzoyl chloride 1565-41-9 C9H7ClO 166.607 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-acetyl-trans-stilbene —— C16H14O 222.287 —— 4-acetylstilbene 3112-03-6 C16H14O 222.287 —— 4-acetyl-4′-methyl-trans-stilbene —— C17H16O 236.313 4-乙酰苯甲醛 p-formylacetophenone 3457-45-2 C9H8O2 148.161 对乙基苯乙酮 4-ethylacetophenone 937-30-4 C10H12O 148.205 —— 1-(4-vinylphenyl)-2-methylpropan-1-one 928758-07-0 C12H14O 174.243 —— (E)-4-(4-acetylphenyl)but-3-en-2-one 115665-94-6 C12H12O2 188.226 —— (E)-1-(4-(oct-1-en-1-yl)phenyl)ethanone 118319-39-4 C16H22O 230.35 —— 4-acetyl-4′-chloro-trans-stilbene 63483-67-0 C16H13ClO 256.732 对氰基苯乙酮 4-cyanophenyl methyl ketone 1443-80-7 C9H7NO 145.161 3-(4-乙酰基苯基)丙醛 3-(4-acetylphenyl)propanal 150805-64-4 C11H12O2 176.215 1-[4-(3-氯丙基)苯基]乙酮 1-[4-(3-chloropropyl)phenyl]ethanone 91427-06-4 C11H13ClO 196.677 —— (E)-4-(2-triethylsilanylvinyl)acetophenone —— C16H24OSi 260.451 —— p-Vinyl-β-dimethylaminopropiophenon 21192-27-8 C13H17NO 203.284 4-乙酰基苯甲酸 4-acetyl-benzoic acid 586-89-0 C9H8O3 164.161 —— (E)-1-(4-(4-methoxystyryl)phenyl)ethanone —— C17H16O2 252.313 —— (2S)-2-(4-acetylphenyl)propanal —— C11H12O2 176.215 —— (2R)-2-(4-acetylphenyl)propanal —— C11H12O2 176.215 —— 4-acetyl-α-methylbenzeneacetaldehyde 63476-27-7 C11H12O2 176.215 —— 4-isopropenylstyrene 16262-48-9 C11H12 144.216 1-(4-环丙基苯基)乙酮 1-(4-cyclopropylphenyl)ethanone 6921-45-5 C11H12O 160.216 3-(4-乙酰基苯基)丙酸 3-(4-acetylphenyl)propanoic acid 39105-51-6 C11H12O3 192.214 —— 3-(4-Acetyl-phenyl)-propionamide 134306-92-6 C11H13NO2 191.23 —— (1S)-1-(4-vinylphenyl)ethanol 1001347-80-3 C10H12O 148.205 —— (1R)-1-(4-vinylphenyl)ethanol 1001347-79-0 C10H12O 148.205 rac-1-(4-乙烯基苯基)乙醇 1-(4-vinylphenyl)ethanol 17250-77-0 C10H12O 148.205 —— [4-carboxy-4'-vinyldibenzoylmethane] 132853-18-0 C18H14O4 294.307 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:1,2-二丁氧基乙烷促进在清洁条件下使用 O2 将烯烃氧化裂解为羧酸摘要:在此,我们报告了第一个在清洁条件下使用 1,2-二丁氧基乙烷/O 2系统将烯烃氧化裂解为羧酸的有效且绿色方法的例子。这种新型氧化系统还具有优异的官能团耐受性,适用于大规模合成。通过一锅式顺序转化,无需外部引发剂、催化剂和添加剂,以良好至极好的收率制备了目标产物。DOI:10.1021/acs.joc.1c01701
-
作为产物:描述:参考文献:名称:Baddeley et al., Journal of the Chemical Society, 1953, p. 2110,2114摘要:DOI:
文献信息
-
Effect of cyclodextrin on elimination reactions作者:Luis Viola、Rita H de RossiDOI:10.1139/v99-085日期:1999.6.1
The reaction of 1-bromo-2-X-2-(Y-phenyl) ethane derivatives (1: X = Y = H; 2: X = Ph, Y = H; 3: X = H, Y = 4-Ac; 4: X = H, Y = 3-NO2; 5: X = H, Y = 4-NO2; 6: X = H, Y = 3-Me; 7: X = H, Y = 4-Me) in basic solution was studied, and in most cases, only the elimination product is formed. Only (2-bromo-1-phenylethyl)benzene, 2, yielded significant substitution product, and this yield decreased with the concentration of HO-. Addition of cyclodextrin (β-CD) diminished (about half for 0.02 M cyclodextrin concentration) the reaction rate of all substrates but 4 and 5. In the latter two cases, the rate rises. The observed rate-constant value at 0.5 M NaOH is 6.78 × 10-4 s-1 (at 40°C) and 1.80 × 10-3 s-1 (at 25°C) for 4 and 5, respectively. Under the same reaction conditions but with 0.01 M β-CD, the corresponding rates were 7.70 × 10-4 s-1 and 5.20 × 10-3 s-1. The elimination yield for 2 increased from 64 to 98% when the β-CD changed from zero to 0.02 M at 0.5 M NaHO. Also, there was an increase in the relative elimination products of 20-40% for compounds 6 and 7. The Hammet ρ values were 1.3 and 2.3 for the reaction in pure solvent and in the presence of β-cyclodextrin, indicating an increase in the negative character of the transition state for the reactions in the latter conditions. The results are interpreted in terms of the formation of an inclusion complex whose structure depends on the substrate.Key words: cyclodextrin, elimination reactions, inhibition, catalysis.
1-溴-2-X-2-(Y-苯基)乙烷衍生物(1:X = Y = H;2:X = Ph,Y = H;3:X = H,Y = 4-Ac;4:X = H,Y = 3-NO2;5:X = H,Y = 4-NO2;6:X = H,Y = 3-Me;7:X = H,Y = 4-Me)在碱性溶液中的反应进行了研究,在大多数情况下,只形成消除产物。只有(2-溴-1-苯基乙基)苯,2,产生了显著的取代产物,这种产量随着HO-浓度的减少而减少。环糊精(β-CD)的添加减少了所有底物的反应速率(对于0.02 M环糊精浓度减少了约一半),但对于4和5而言,速率却上升了。在后两种情况下,速率增加。在0.5 M NaOH下观察到的速率常数值分别为6.78 × 10-4 s-1(在40°C下)和1.80 × 10-3 s-1(在25°C下)对于4和5。在相同的反应条件下,但使用0.01 M β-CD,相应的速率分别为7.70 × 10-4 s-1和5.20 × 10-3 s-1。当β-CD从零变为0.02 M时,2的消除产量从64%增加到98%。此外,化合物6和7的相对消除产物增加了20-40%。在纯溶剂和β-环糊精存在的情况下,Hammet ρ值分别为1.3和2.3,表明在后一种条件下反应的过渡态的负性特征增加。结果根据形成取决于底物的包含复合物的结构进行解释。关键词:环糊精,消除反应,抑制,催化。 -
Pd-Catalyzed Vinylation of Aryl Halides with Inexpensive Organosilicon Reagents Under Mild Conditions作者:Chu-Ting Yang、Jun Han、Jun Liu、Yi Li、Fan Zhang、Hai-Zhu Yu、Sheng Hu、Xiaolin WangDOI:10.1002/chem.201802573日期:2018.7.20Pd‐catalyzed Hiyama vinylation reaction of non‐activated aryl chlorides and bromides under mild conditions was developed. The use of efficient vinyl donors and electron‐rich sterically hindered phosphine ligands was critical for the success of the reaction. The products of this transformation can be used for Am/Cm separation, an important challenge in nuclear fuel reprocessing. The substituent effect
-
Model Guided Development of a Simple Catalytic Method for the Synthesis of Unsymmetrical Stilbenes by Sequential Heck Reactions of Aryl Bromides with Ethylene作者:Helen Barlow、Jonas Y. Buser、Hendrik Glauninger、Carla V. Luciani、Joseph R. Martinelli、Niall Oram、Nichole Thompson‐Van Hook、Jeffery RichardsonDOI:10.1002/adsc.201800167日期:2018.7.16moieties, but methods for their preparation typically possess numerous inefficiencies. Presented here is a methodology for the two‐step, one pot preparation of unsymmetrical stilbenes via sequential Heck reactions. The first Heck reaction with ethylene gas was analysed as a function of temperature and pressure for electronically differentiated naphthyl bromides and model‐aided reaction optimization was utilized
-
Reaktionen polyvalenter jodverbindungen—III作者:J. Ehrenfreund、E. ZbiralDOI:10.1016/0040-4020(72)88050-5日期:1972.1Pb(OAc)4(CH3)3SiN3 reacts with simple nucleophilic olefins to form mostly diazides and azide-acetoxy compounds,2 while with steroidal olefines α-azidosteroidketones3 are afforded. Contrary to this result the title reagent reacts not only with nucleophilic but also with electrophilic double-bounds yielding α-azido-carbonyl compounds. Not any azide-acetoxy compound is formed. Compound 14 indicates that
-
Synthesis of α-Fluoroketones from Vinyl Azides and Mechanism Interrogation作者:Shu-Wei Wu、Feng LiuDOI:10.1021/acs.orglett.6b01691日期:2016.8.5An efficient and mild fluorination of vinyl azides for the synthesis of α-fluoroketones is described. The mechanistic studies indicated that a single-electron transfer (SET) and a subsequent fluorine atom transfer process could be involved in the reaction.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
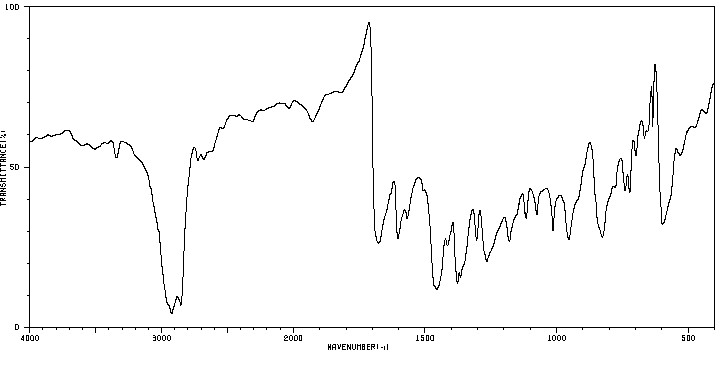
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息