2-萘甲醇 | 1592-38-7
中文名称
2-萘甲醇
中文别名
Β-萘甲醇;β-萘甲醇
英文名称
2-Naphthalenemethanol
英文别名
naphthalen-2-ylmethanol;2-naphthylmethanol;2-naphthylcarbinol;2-naphthalenmethanol
CAS
1592-38-7
化学式
C11H10O
mdl
——
分子量
158.2
InChiKey
MFGWMAAZYZSWMY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:79-81 °C (lit.)
-
沸点:178°C/12mmHg(lit.)
-
密度:0.9887 (rough estimate)
-
溶解度:可溶于乙腈(少许)、氯仿(少许)
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:12
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.09
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险等级:IRRITANT
-
危险品标志:F,Xi,C
-
安全说明:S24/25
-
危险类别码:R11
-
WGK Germany:3
-
海关编码:2906299090
-
危险品运输编号:NONH for all modes of transport
-
危险类别:IRRITANT
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:请将容器密封保存,并存放在阴凉、干燥处。
SDS
| Name: | 2-Naphthalenemethanol 98% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 1592-38-7 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1592-38-7 | 2-Naphthalenemethanol | 98% | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. May be harmful if inhaled.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
In case of fire, use carbon dioxide, dry chemical powder or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1592-38-7: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white to yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 79 - 81 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C11H10O
Molecular Weight: 158.20
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not currently available.
Conditions to Avoid:
Incompatible materials, dust generation.
Incompatibilities with Other Materials:
Acids, acid chlorides, acid anhydrides, oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1592-38-7 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-Naphthalenemethanol - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 1592-38-7: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1592-38-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1592-38-7 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— O-(naphthalen-2-ylmethyl)hydroxylamine 54484-69-4 C11H11NO 173.214 1-萘甲醇 1-naphthalene methanol 4780-79-4 C11H10O 158.2 2-甲基萘 2-Methylnaphthalene 91-57-6 C11H10 142.2 2-萘甲酸 2-Naphthoic acid 93-09-4 C11H8O2 172.183 1-(2-萘甲酰基 )乙醇 1-(2-naphthyl)ethanol 7228-47-9 C12H12O 172.227 —— 2-(dimethoxymethyl)naphthalene 77196-31-7 C13H14O2 202.253 萘-2-基甲基乙酸酯 2-(acetoxymethyl)naphthalene 35480-23-0 C13H12O2 200.237 2-萘甲醛 β-naphthaldehyde 66-99-9 C11H8O 156.184 2-萘甲酸甲酯 methyl naphthalene-2-carboxylate 2459-25-8 C12H10O2 186.21 2-萘甲腈 2-Cyanonaphthalene 613-46-7 C11H7N 153.183 2-(氯甲基)萘 2-chloromethylnaphthalene 2506-41-4 C11H9Cl 176.645 2-溴甲基萘 2-bromomethylnaphthyl bromide 939-26-4 C11H9Br 221.096 6-溴-2-萘甲酸 6-bromo-2-naphthoic acid 5773-80-8 C11H7BrO2 251.079 —— 2-bromo-3-(hydroxymethyl)naphthalene 38399-19-8 C11H9BrO 237.096 —— 2-(2-naphthyl)dioxolane 4469-45-8 C13H12O2 200.237 2-萘甲酸乙酯 ethyl-2-naphthoate 3007-91-8 C13H12O2 200.237 1H-环丙并[b]萘 1H-cyclopropa[b]naphthalene 286-85-1 C11H8 140.185 —— triethyl(naphthalen-2-ylmethoxy)silane —— C17H24OSi 272.462 6-溴-2-萘甲酸甲酯 methyl 6-bromo-2-naphthoate 33626-98-1 C12H9BrO2 265.106 —— naphthalen-2-ylmethyl trifluoroacetate —— C13H9F3O2 254.208 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-萘基甲基甲基醚 2-(methoxymethyl)naphthalene 42101-92-8 C12H12O 172.227 —— O-(naphthalen-2-ylmethyl)hydroxylamine 54484-69-4 C11H11NO 173.214 2-(乙氧基甲基)萘 2-((vinyloxy)methyl)naphthalene 91902-40-8 C13H12O 184.238 —— 2-naphthylmethoxymethyl chloride 914300-10-0 C12H11ClO 206.672 —— bis[(2-naphthyl)methyl] ether 55644-75-2 C22H18O 298.384 2-甲基萘 2-Methylnaphthalene 91-57-6 C11H10 142.2 2-萘甲酸 2-Naphthoic acid 93-09-4 C11H8O2 172.183 —— (2-naphthylmethyl) propargyl ether 109930-24-7 C14H12O 196.249 —— 2-naphthylmethyl chloroformate 141700-30-3 C12H9ClO2 220.655 —— naphthalen-2-ylmethyl carbamate 1026440-01-6 C12H11NO2 201.225 —— 4-(2-naphthylmethyloxy)-2Z-butene-1-ol 1283072-99-0 C15H16O2 228.291 萘-2-基甲基乙酸酯 2-(acetoxymethyl)naphthalene 35480-23-0 C13H12O2 200.237 2-萘甲醛 β-naphthaldehyde 66-99-9 C11H8O 156.184 —— 2-(naphthalen-2-ylmethoxy)acetic acid —— C13H12O3 216.236 2-萘甲酸甲酯 methyl naphthalene-2-carboxylate 2459-25-8 C12H10O2 186.21 —— 2-(mercaptomethyl)naphthalene 1076-67-1 C11H10S 174.266 2-乙烯基萘 2-Vinylnaphthalene 827-54-3 C12H10 154.211 萘-2-甲胺 naphthalen-2-ylmethylamine 2018-90-8 C11H11N 157.215 2-(碘甲基)萘 2-(iodomethyl)naphthalene 24515-49-9 C11H9I 268.097 —— 2-(fluoromethyl)naphthalene 55831-11-3 C11H9F 160.191 2-乙基萘 2-ethylnaphthalene 939-27-5 C12H12 156.227 2-萘甲腈 2-Cyanonaphthalene 613-46-7 C11H7N 153.183 2-(氯甲基)萘 2-chloromethylnaphthalene 2506-41-4 C11H9Cl 176.645 2-溴甲基萘 2-bromomethylnaphthyl bromide 939-26-4 C11H9Br 221.096 —— bromoacetic acid 2-naphthalenylmethyl ester —— C13H11BrO2 279.133 —— 2-naphthylmethyl acrylate 93359-80-9 C14H12O2 212.248 3-(羟基甲基)萘-2-醇 3-hydroxymethyl-2-naphthol 30159-70-7 C11H10O2 174.199 萘-2-基(苯基)甲醇 (2-naphthyl)phenylmethanol 35060-38-9 C17H14O 234.298 2-(苯氧基甲基)萘 β-naphthylmethyl phenyl ether 68299-58-1 C17H14O 234.298 —— (R)-2-methoxy-3-(2-naphthylmethoxy)-1-propanol 252272-41-6 C15H18O3 246.306 —— 2-naphthylmethyl pivalate —— C14H15NO2 229.279 1-(萘-2-基)-丁-3-烯-1-醇 1-(2-naphthyl)-3-buten-1-ol 76635-86-4 C14H14O 198.265 2-萘乙醇 naphthalen-2-ethanol 1485-07-0 C12H12O 172.227 —— naphthalen-2-ylmethyl benzoate 38418-15-4 C18H14O2 262.308 —— triethyl(naphthalen-2-ylmethoxy)silane —— C17H24OSi 272.462 —— (E)-2-(prop-1-en-1-yl)naphthalene 195319-72-3 C13H12 168.238 —— 2-(prop-1-en-1-yl)naphthalene 263911-05-3 C13H12 168.238 —— 2-naphthaldoxime 24091-02-9 C11H9NO 171.199 —— (E)-2-naphthaldehyde oxime 51873-98-4 C11H9NO 171.199 —— 2-(difluoromethyl)naphthalene 140135-76-8 C11H8F2 178.181 2-萘乙腈 2-naphthaleneacetonitrile 7498-57-9 C12H9N 167.21 —— (S)-1-bromo-2-methoxy-3-(2-naphthylmethoxy)propane 252272-47-2 C15H17BrO2 309.203 萘-1-基甲基乙酸酯 naphthalen-1-ylmethyl acetate 13098-88-9 C13H12O2 200.237 —— N,N-dimethyl-1-(naphthalen-2-yl)methanamine 2018-89-5 C13H15N 185.269 —— naphthalen-2-ylmethyl 2-naphthoate 135512-98-0 C22H16O2 312.368 2-苄基萘 2-benzylnaphthalene 613-59-2 C17H14 218.298 —— naphthalen-2-ylmethyl pivalate 184424-59-7 C16H18O2 242.318 2-萘丙醇 3-(2-naphthyl)propan-1-ol 27650-98-2 C13H14O 186.254 2-(叠氮甲基)萘 2-azidomethylnaphthalene 164269-42-5 C11H9N3 183.213 —— cis-1,2-bis(2-naphthyl)ethylene 2633-08-1 C22H16 280.369 —— (E)-2-styrylnaphthalene —— C18H14 230.309 —— 1,2-di-[2]naphthyl-ethene 2042-99-1 C22H16 280.369 双(2-萘基甲基)胺 bis(naphthalen-2-ylmethyl)amine 47304-99-4 C22H19N 297.4 —— N-benzyl-1-(naphthalen-2-yl)methanamine 46995-63-5 C18H17N 247.34 丙基萘-2-羧酸酯 propyl 2-naphthoate 3007-90-7 C14H14O2 214.264 —— naphthalen-2-ylmethyl trifluoroacetate —— C13H9F3O2 254.208 - 1
- 2
- 3
- 4
- 5
- 6
反应信息
-
作为反应物:参考文献:名称:在没有额外碱的简单条件下,可回收的Pd / C催化羰基一步还原为烃摘要:分别用水合肼,氢气和甲酸铵开发了用于合成烃的羰基还原剂。简单的,温和和有效的条件进行了通过采用可回收利用Pd / C作为在100正常溶剂催化剂提供ö C和平稳地进行,以产生相应的产品具有良好到优异的产率的反应。并证明了克级反应和催化剂的循环利用。此外,已经提出了该机制。DOI:10.1016/j.tetlet.2019.151447
-
作为产物:参考文献:名称:取代的芳族烃的选择性氧化和脱开的观察通过由CYP101B1系统萘氧化期间氧化还原循环†摘要:细胞色素P450单加氧酶CYP101B1,来自Novosphingobium芳烃DSM12444有效且选择性地氧化了各种萘和联苯衍生物。甲基取代的萘是比乙基萘和萘本身更好的底物。用2-甲基萘获得单取代烷基萘的最高产物形成活性。烷基萘的氧化对苄基甲基或次甲基CH键具有区域选择性。来自1-和2-乙基萘氧化的产物是高度对映体选择性的,并且明显过量地产生了单个立体异构体。与1-和2-甲基萘相比,双取代的底物2,7-二甲基萘具有更高的产物形成活性。甲基取代的联苯也是比联苯更好的底物,并且具有与1-甲基萘相似的生物催化参数。CYP101B1催化的2-和3-甲基联苯的氧化对甲基CH键具有选择性。例外的是4-甲基联苯的周转量,它以4'-(4-甲基苯基)苯酚为主要产物(占70%),其余部分由4-联苯甲醇构成。药物分子双氯芬酸也被CYP101B1区域选择性氧化为4'-羟基双氯芬酸。带有萘的CYP101B1系统DOI:10.1039/c7cy00088j
文献信息
-
B(C<sub>6</sub>F<sub>5</sub>)<sub>3</sub>-Catalyzed Hydrodesulfurization Using Hydrosilanes – Metal-Free Reduction of Sulfides作者:Kodai Saito、Kazumi Kondo、Takahiko AkiyamaDOI:10.1021/acs.orglett.5b01651日期:2015.7.2B(C6F5)3-catalyzed hydrodesulfurization of carbon–sulfur bonds was achieved using triethylsilane as the reducing agent. The corresponding products were obtained in good yields under mild reaction conditions. This protocol could be applied to the reduction of sulfides, including benzyl and alkyl sulfides and dithianes, with high chemoselectivities.
-
A Bifunctional Copper Catalyst Enables Ester Reduction with H<sub>2</sub>: Expanding the Reactivity Space of Nucleophilic Copper Hydrides作者:Birte M. Zimmermann、Trung Tran Ngoc、Dimitrios-Ioannis Tzaras、Trinadh Kaicharla、Johannes F. TeichertDOI:10.1021/jacs.1c09626日期:2021.10.13activation of esters through hydrogen bonding and formation of nucleophilic copper(I) hydrides from H2, resulting in a catalytic hydride transfer to esters. The reduction step is further facilitated by a proton shuttle mediated by the guanidinium subunit. This bifunctional approach to ester reductions for the first time shifts the reactivity of generally considered “soft” copper(I) hydrides to previously
-
Clean and selective oxidation of aromatic alcohols using silica-supported Jones’ reagent in a pressure-driven flow reactor作者:Charlotte Wiles、Paul Watts、Stephen J. HaswellDOI:10.1016/j.tetlet.2006.05.157日期:2006.7By exploiting the high surface to volume ratio obtained within continuous flow reactors, we are able to oxidise selectively an array of primary alcohols to either the aldehyde or carboxylic acid, depending on the flow rates employed, demonstrating a degree of reaction control unattainable in traditional stirred reactors.通过利用连续流动反应器中获得的高表面积体积比,我们能够根据所使用的流速将一系列伯醇选择性地氧化为醛或羧酸,证明了在传统搅拌条件下无法实现的反应控制程度反应堆。
-
Selective aerobic oxidation of allylic and benzylic alcohols catalyzed by N-hydroxyindole and copper(I) chloride作者:Shu-Su Shen、Vita Kartika、Ying Shan Tan、Richard D. Webster、Koichi NarasakaDOI:10.1016/j.tetlet.2011.12.058日期:2012.21-hydroxy-2-methyl-6-trifluoromethyl-1H-indole-3-carboxylate acted as a catalyst for the chemoselective aerobic oxidation of allylic and benzylic alcohols. A variety of primary and secondary allylic and benzylic alcohols were oxidized into the corresponding α,β-unsaturated carbonyl compounds in good yields without affecting non-allylic alcohols.
-
A general and practical oxidation of alcohols to primary amides under metal-free conditions作者:Xiao-Feng Wu、Muhammad Sharif、Jian-Bo Feng、Helfried Neumann、Anahit Pews-Davtyan、Peter Langer、Matthias BellerDOI:10.1039/c3gc40668g日期:——A general procedure for oxidation of both benzyl alcohols and alkyl alcohols to primary amides under catalyst free conditions has been developed. 34 examples of primary amides were produced from their corresponding alcohols in moderate to excellent yields. This is a practical procedure for primary amides synthesis; water and tert-butanol are the only by-products. A commercial drug, Piracetam, was prepared
表征谱图
-
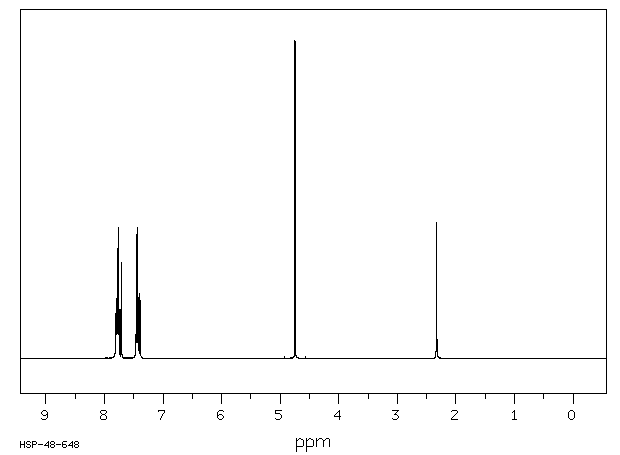
氢谱1HNMR
-
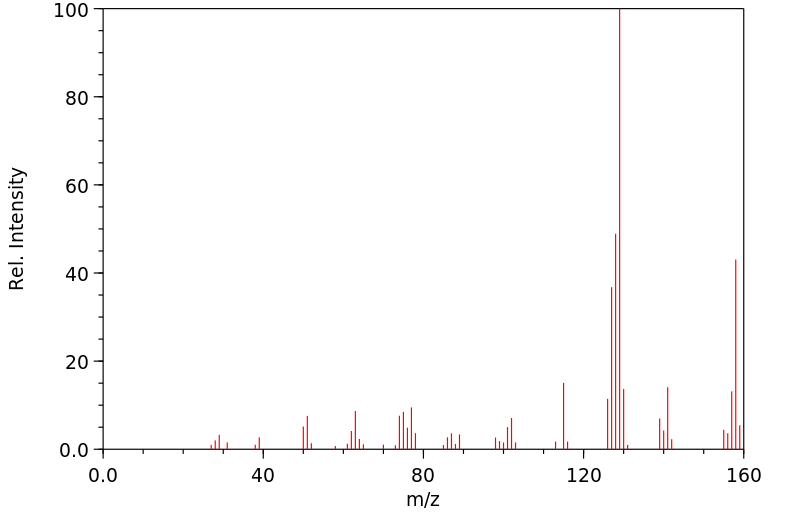
质谱MS
-
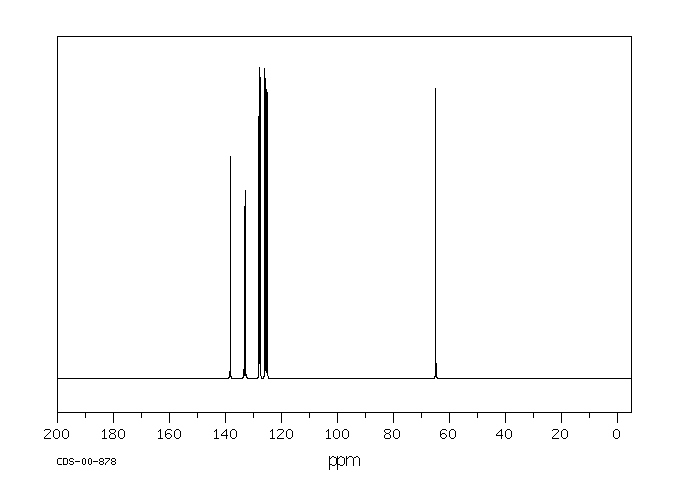
碳谱13CNMR
-
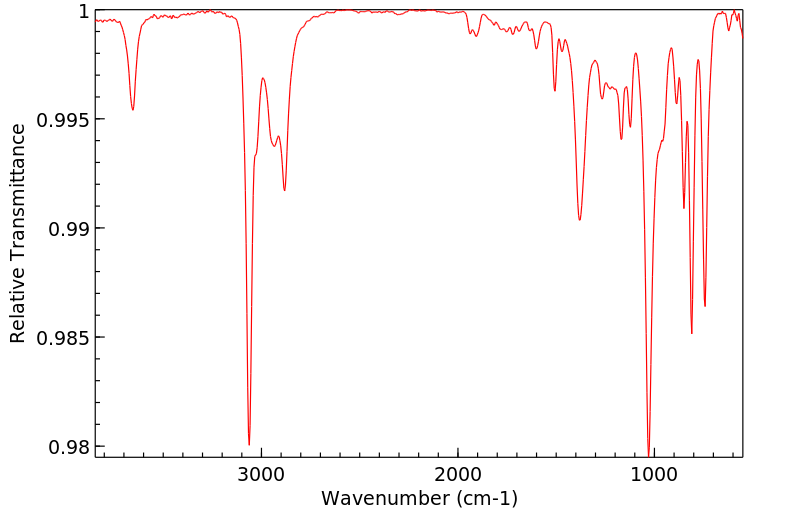
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮