1H-环丙并[b]萘 | 286-85-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:11
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.09
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902909090
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:萘[b]环丙烯反应的研究摘要:为了研究萘[b]环丙烯(2)的反应性,检查了与各种亲二烯体,二烯和亲电体的反应。的反应2与四氰基乙烯,4-苯基-3H-1,2,4-三唑-3,5(4H) -二酮,1,3- diphenylisobenzofuran和苯炔,得到插入的产品3,4,6,10。Cheletropic除了二氯卡宾至2,得到1,1- dichloronaphtho并[b]环丁烯(11)。水解和还原消除12种提供的萘[b]环丁烯酮(13)和萘[b]环丁烯(13)), 分别。萘[b]环丙烯与不同的亲电子试剂(例如乙酸,HCl,马来酸,碘,溴,乙醇和氢)反应,分别得到相应的开环产物14、15、16、17、18、19、20和21。DOI:10.1016/0040-4020(95)00653-p
-
作为产物:参考文献:名称:萘[b]环丙烯反应的研究摘要:为了研究萘[b]环丙烯(2)的反应性,检查了与各种亲二烯体,二烯和亲电体的反应。的反应2与四氰基乙烯,4-苯基-3H-1,2,4-三唑-3,5(4H) -二酮,1,3- diphenylisobenzofuran和苯炔,得到插入的产品3,4,6,10。Cheletropic除了二氯卡宾至2,得到1,1- dichloronaphtho并[b]环丁烯(11)。水解和还原消除12种提供的萘[b]环丁烯酮(13)和萘[b]环丁烯(13)), 分别。萘[b]环丙烯与不同的亲电子试剂(例如乙酸,HCl,马来酸,碘,溴,乙醇和氢)反应,分别得到相应的开环产物14、15、16、17、18、19、20和21。DOI:10.1016/0040-4020(95)00653-p
文献信息
-
[2p+2s] Type Cycloaddition Reactions of Iminotropone Derivatives with Naphtho[b]cyclopropene to Form Cyclic Amine Compounds作者:Katsuhiro Saito、Katsuhiko Ono、Narie Ito、Naoe Tada、Shinichi AndoDOI:10.3987/com-01-9416日期:——Reactions of iminotropone derivatives with naphtho[b]cyclopropene under the presence of a catalytic amount of AgBF 4 afforded cyclic amine derivatives via [2 π + 2 σ] type cycloaddition reactions. On the other hand, a reaction using a tropone hydrazone derivative without a catalysis formed a substituted hydrazone via a σ -bond rupture of the cyclopropene ring.
-
Synthesis of cycloproparenes<i>via</i>aromatization of 7-Oxanorbornenes with Low-Valent titanium作者:Paul Müller、Jean-Pierre SchallerDOI:10.1002/hlca.19890720721日期:1989.11.11H-Cyclopropa[b]naphthalene 3c and the 2,7-diphenyl-substituted derivative 3a have been synthesized via cycloaddition of the appropriate isobenzofurans 1a and 1b to 1-bromo-2-chlorocyclopropene and aromatization of the adducts with low-valent Ti. The same procedure afforded the 2,7-dimethyl-H-cyelopropa[g]isoquinoline (15), but failed for the parent azacompound. Reaction of adducts of furans to 1-通过将适当的异苯并呋喃1a和1b环加成至1-溴-2-氯环丙烯并用低价Ti进行加合物的芳构化反应,合成了1 H-环丙烷[ b ]萘3c和2,7-二苯基取代的衍生物3a。。相同的步骤,得到2,7-二甲基- ħ -cyelopropa [克]异喹啉(15),但未能父azacompound。呋喃与1-溴-2-氯环丙烯的加合物与低价Ti生成的环丙烷苯19和1,6-二卤代-1,3,5-环庚烷18的混合物反应。后者可以用BuLi转化为环丙苯。
-
Studies in the Cycloproparene Series: Cycloaddition Reactions With 1,3-Diphenylisobenzofuran作者:B Halton、SGG RussellDOI:10.1071/ch9902099日期:——
The behaviour of cyclopropabenzene (1) with 1,3-diphenylisobenzofuran ( dpibf ) is temperature dependent. Addition to the strained bridge bond of (1) gives the Diels -Alder adduct (13) in tetrahydrofuran at 20°C, but in chloroform at the same temperature the ring-expanded 5,10-epoxydibenzocycloheptene (12) is also formed. Compound (12) becomes the sole product from reaction at 30°C or higher. Cyclopropa [b]naphthalene (2) is inert to dpibf under similar conditions but provides the dimer 6,13-dihydropentacene (15). The triazoloindazole (19) results from reaction of (2) with 4-phenyl-3H-1,2,4-triazole-3,5(4H)- dione.
-
Methylidenecycloproparenes: Novel Compounds with Fascinating Properties作者:Brian Halton、Peter StangDOI:10.1055/s-1997-723日期:1997.2Proton abstraction from the methylene position of a cycloproparene provides the corresponding C1 anion that can be intercepted by a carbonyl-containing compound to provide a wide range of novel methylidenecycloproparenes. The physical and chemical aspects of this comparatively new class of surprisingly stable, strained compounds have been explored. The present account provides a perspective on these developments from the initial experiments in the Utah laboratories.
-
Studies in the Cycloproparene Series: Electron Transfer Induced Dimerizations of Cyclopropa[b]naphthalene作者:MJ Cooney、B Halton、M Baumgarten、L GherghelDOI:10.1071/ch9951167日期:——
Attempts to characterize the radical anion from 1H-cyclopropa[b]naphthalene (1) by conventional electron transfer techniques has led instead to detection of the pentacene radical anion (10)•- and the pentaphene analogue (11)•- by ENDOR spectroscopy. Reactions of (1) with potassium in tetrahydrofuran on a preparative scale depend upon the surface, and 6,13-dihydropentacene (8), 6,7-dihydropentaphene (9), pentacene (10), 2-methylnaphthalene (12) and 1,2-di(2′-naphthyl)ethane (13) are formed.
表征谱图
-
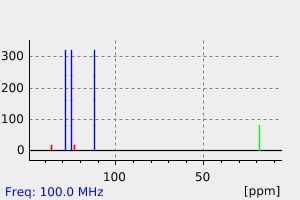
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息