3,5-二叔丁基苯胺 | 2380-36-1
中文名称
3,5-二叔丁基苯胺
中文别名
3,5-DI-叔-丁基苯胺
英文名称
3,5-di-tert-butylaniline
英文别名
3,5-Di-tert-butylanilin;stopper amine;3,5-ditert-butylaniline
CAS
2380-36-1
化学式
C14H23N
mdl
MFCD00134096
分子量
205.343
InChiKey
MJKNHXCPGXUEDO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:54-57 °C (lit.)
-
沸点:276.6±29.0 °C(Predicted)
-
密度:0.912±0.06 g/cm3(Predicted)
-
闪点:>230 °F
-
溶解度:溶于苯、醇
计算性质
-
辛醇/水分配系数(LogP):4.6
-
重原子数:15
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.571
-
拓扑面积:26
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:29214990
-
危险性防范说明:P261,P264,P270,P271,P280,P301+P312+P330,P302+P352+P312,P304+P340+P312,P305+P351+P338,P332+P313,P337+P313,P362+P364,P501
-
危险性描述:H302+H312+H332,H315,H319
-
储存条件:| 室温 干燥 |
SDS
3,5-Di-tert-butylaniline Revision number: 6
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 3,5-Di-tert-butylaniline
Revision number: 6
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Acute toxicity (Oral) Category 4
Category 4
Acute toxicity (Dermal)
Acute toxicity (Inhalation) Category 4
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Harmful if swallowed, in contact with skin or if inhaled
Hazard statements
Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Avoid breathing dust/fume/gas/mist/vapours/spray.
[Prevention]
Use only outdoors or in a well-ventilated area.
Do not eat, drink or smoke when using this product.
Wash hands thoroughly after handling.
Wear protective gloves and eye/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing. Call a POISON CENTER or doctor/physician if you feel unwell.
IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
Rinse mouth.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Wash contaminated clothing before reuse.
Call a POISON CENTER or doctor/physician if you feel unwell.
3,5-Di-tert-butylaniline
Section 2. HAZARDS IDENTIFICATION
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 3,5-Di-tert-butylaniline
>98.0%(GC)
Percent:
CAS Number: 2380-36-1
Chemical Formula: C14H23N
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Call a POISON CENTER or doctor/physician if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Rinse cautiously with water for several minutes. Remove contact lenses, if present
Eye contact:
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Call a POISON CENTER or doctor/physician if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
from the chemical:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Air-sensitive
Comply with laws.
Packaging material:
3,5-Di-tert-butylaniline
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Protective gloves.
Hand protection:
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
White - Pale reddish yellow
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:55°C
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
No data available
[Water]
[Other solvents]
Soluble: Alcohols, Benzene, Hexane
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
3,5-Di-tert-butylaniline
Section 12. ECOLOGICAL INFORMATION
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 3,5-Di-tert-butylaniline
Revision number: 6
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Acute toxicity (Oral) Category 4
Category 4
Acute toxicity (Dermal)
Acute toxicity (Inhalation) Category 4
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Harmful if swallowed, in contact with skin or if inhaled
Hazard statements
Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Avoid breathing dust/fume/gas/mist/vapours/spray.
[Prevention]
Use only outdoors or in a well-ventilated area.
Do not eat, drink or smoke when using this product.
Wash hands thoroughly after handling.
Wear protective gloves and eye/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing. Call a POISON CENTER or doctor/physician if you feel unwell.
IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
Rinse mouth.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Wash contaminated clothing before reuse.
Call a POISON CENTER or doctor/physician if you feel unwell.
3,5-Di-tert-butylaniline
Section 2. HAZARDS IDENTIFICATION
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 3,5-Di-tert-butylaniline
>98.0%(GC)
Percent:
CAS Number: 2380-36-1
Chemical Formula: C14H23N
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Call a POISON CENTER or doctor/physician if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Rinse cautiously with water for several minutes. Remove contact lenses, if present
Eye contact:
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Call a POISON CENTER or doctor/physician if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
from the chemical:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Air-sensitive
Comply with laws.
Packaging material:
3,5-Di-tert-butylaniline
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Protective gloves.
Hand protection:
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
White - Pale reddish yellow
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:55°C
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
No data available
[Water]
[Other solvents]
Soluble: Alcohols, Benzene, Hexane
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
3,5-Di-tert-butylaniline
Section 12. ECOLOGICAL INFORMATION
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 3,5-di-tert-butylacetanilide 37055-54-2 C16H25NO 247.381 3,5-二叔丁基甲苯 3, 5-di-tert-butyltoluene 15181-11-0 C15H24 204.356 3,5-二叔丁基苄醇 3,5-di-tert-butylbenzyl alcohol 77387-57-6 C15H24O 220.355 3,5-二叔丁基苯酚 3,5-Di-tert-butylphenol 1138-52-9 C14H22O 206.328 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3,5-di-t-butylphenylhydrazine 131925-97-8 C14H24N2 220.358 —— 3,5-di-tert-butyl-N-methylaniline 224631-95-2 C15H25N 219.37 —— 3,5-di-t-butyl-nitrosobenzene 18628-44-9 C14H21NO 219.327 —— 3,5-di-tert-butyldimethylaniline 53172-38-6 C16H27N 233.397 —— 1,3-di-tert-butyl-5-isothiocyanatobenzene 259222-92-9 C15H21NS 247.404 —— 3,5-di-tert-butylphenyl isocyanate 954407-25-1 C15H21NO 231.338 —— bis-3,3’,5,5’-tetra-tert-butyldiphenylamine 37055-49-5 C28H43N 393.656 —— 1-azido-3,5-di-tert-butylbenzene 1275593-72-0 C14H21N3 231.341 —— 3,3',5,5'-tetra-t-butylazobenzene 80585-17-7 C28H42N2 406.655 —— N,N'-bis(3,5-di-tert-butylphenyl)ethylenediamine 935476-38-3 C30H48N2 436.725 —— 1,4-bis(3,5-di-tert-butylphenyl)-1,4-diaza-1,3-butadiene 404373-30-4 C30H44N2 432.693 —— 1,4-bis(3,5-di-tert-butylphenyl)-1,4-diaza-1,3-butadiene —— C30H44N2 432.693 —— N-(2,2-dimethylpropylidene)-3,5-bis(1,1-dimethylethyl)aniline —— C19H31N 273.462 —— N-(t-butylthio)-3,5-di-t-butylaniline —— C18H31NS 293.517 —— 3,5-di-tert-butylacetanilide 37055-54-2 C16H25NO 247.381 —— 3,5-Di-t-butyl-1-nitrobenzol 38896-15-0 C14H21NO2 235.326 —— N-Methylsulfinyl-3,5-di-t-butylaniline 128729-18-0 C15H25NOS 267.436 2,6-二-叔-丁基苯胺 2,6-Di-tert-butyl-anilin 2909-83-3 C14H23N 205.343 —— N-(3,5-di-tert-butylphenyl)methanesulfonamide 66651-44-3 C15H25NO2S 283.435 1,1-双(3,5-二叔丁基苯基)肼 1,1-bis(3,5-di-tert-butylphenyl)hydrazine 55166-13-7 C28H44N2 408.671 —— 1-(3,5-di-tert-butylphenyl)piperazine —— C18H30N2 274.45 —— 2-Mercapto-3,5-di-tbutylanilin 112548-22-8 C14H23NS 237.409 —— N,N'-bis(3,5-di-tert-butylphenyl) oxamide 156777-89-8 C30H44N2O2 464.692 —— 1-(3,5-Ditert-butylphenyl)-3-[4-(hydroxymethyl)phenyl]urea 1464148-73-9 C22H30N2O2 354.492 —— 3,5-ditert-butyl-N-[(1S)-1-phenylethyl]aniline 928789-92-8 C22H31N 309.495 —— 2,6-dibromo-3,5-di-tert-butylaniline —— C14H21Br2N 363.135 1,3-二叔丁基-5-碘苯 1-iodo-3,5-di-tert-butylbenzene 37055-53-1 C14H21I 316.225 1,3-二叔丁基-5-氯苯 1-chloro-3,5-di-tert-butylbenzene 80438-67-1 C14H21Cl 224.774 3,5-二叔丁基苯酚 3,5-Di-tert-butylphenol 1138-52-9 C14H22O 206.328 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:具有仿生 SCS 钳形配体的铁配合物的合成和反应性摘要:最近的实验证据表明,固氮酶的 FeMoco 在 N 2还原过程中经历了结构重排,这可能导致产生具有两个硫供体和一个碳供体的配位不饱和铁位点。为了合成和研究具有一碳/二硫配位环境的小分子模型配合物,我们设计了两种新的 SCS 钳形配体,其中含有一个中心 NHC 供体,并伴有硫醚或硫醇官能化的芳基。硫醚配体与 Fe(OTf) 2的金属化得到 6 配位配合物,其中 SCS 配体经向结合。相反,硫醇配体与 Fe(HMDS) 2的金属化给出了一个四坐标假四面体酰胺复合物,其中配体表面结合,说明了这些配体的潜在结构灵活性。酰胺配合物与庞大的单硫醇反应产生四配位配合物,其具有类似于固氮酶的静止状态的一碳/三硫配位环境。酰胺络合物与苯肼反应得到具有稀有 κ 1键合苯肼基的产物,该基团经历 N-N 裂解得到苯酰胺络合物。DOI:10.1021/acs.inorgchem.0c03427
-
作为产物:描述:2,4-二叔丁基苯酚 在 全氟丁基磺酰氟 、 三(三甲基硅)胺 、 双(三甲基硅烷基)氨基钾 、 盐酸 作用下, 以 四氢呋喃 、 水 为溶剂, 反应 5.08h, 以77%的产率得到3,5-二叔丁基苯胺参考文献:名称:一锅苯酚生成苯甲酰苯:通过苯酚的脱氧胺化作用形成初级苯胺。摘要:由苯酚选择性地原位生成苯并在酸性处理后被六甲基二硅氮合钾区域选择性地捕获以形成伯苯胺。酚羟基直接转化为游离氨基是用于制备伯芳基胺的有用方法,该伯芳基胺难以使用涉及苯酚衍生物与氨的偶联反应来合成。虽然邻位和间位取代的苯酚的反应仅产生间位取代的苯胺,但对位取代的苯酚的反应提供了邻位甲硅烷基苯胺。DOI:10.1002/chem.201904987
文献信息
-
Five additional macrocycles that allow Na<sup>+</sup> ion-templated threading of guest units featuring a single urea or amide functionality作者:You-Han Lin、Chien-Chen Lai、Sheng-Hsien ChiuDOI:10.1039/c3ob42418a日期:——Five analogues of the macrocycle BPX26C6 are also capable of recognizing single urea and/or amide functionalities in the presence of templating Na+ ions. We have unambiguously confirmed the formation of such [2]pseudorotaxane complexes in solution through syntheses of the corresponding [2]rotaxanes.大环BPX26C6的五个类似物在模板化的Na +离子存在下也能够识别单个尿素和/或酰胺官能团。我们已经通过相应的[2]轮烷的合成清楚地确认了这种[2]假轮烷的络合物的形成。
-
ORGANIC LIGHT EMITTING ELEMENT, DISPLAY DEVICE, IMAGE INFORMATION PROCESSING DEVICE, LIGHTING DEVICE, IMAGE FORMING DEVICE, EXPOSURE DEVICE, AND ORGANIC PHOTOELECTRIC CONVERSION ELEMENT申请人:CANON KABUSHIKI KAISHA公开号:US20170012215A1公开(公告)日:2017-01-12The present disclosure provides an organic light emitting element which has a pair of electrodes and an organic compound layer disposed therebetween and in which the organic compound layer contains an organic compound represented by the following general formula [1], wherein in the formula [1], Ar 1 and Ar 2 each independently represent an aromatic hydrocarbon group or a heteroaromatic ring group, R 1 to R 4 are each independently selected from a hydrogen atom or a substituent, R 1 and R 2 and R 3 and R 4 each may form a benzene ring, wherein the benzene ring may have at least one substituent.本公开提供了一种有一对电极和位于其之间的有机化合物层的有机发光元件,其中所述有机化合物层包含由下述通用式[1]表示的有机化合物, 在通用式[1]中,Ar1和Ar2分别独立表示芳香烃基或杂芳环基,R1至R4分别独立地选自氢原子或取代基,R1和R2以及R3和R4各自可以形成苯环,其中苯环可以具有至少一个取代基。
-
[EN] COMPOSITIONS AND METHODS FOR IMMUNE MODULATION AND TREATMENT OF CANCER<br/>[FR] COMPOSITIONS ET MÉTHODES DE MODULATION IMMUNITAIRE ET DE TRAITEMENT DU CANCER申请人:UNIV MICHIGAN STATE公开号:WO2020150668A1公开(公告)日:2020-07-23The disclosure relates to rexinoids, including compounds of the Formula (I) and (II) or a pharmaceutically acceptable salt, polymorph, prodrug, solvate or clathrate thereof. These rexinoids are useful for increasing PD-L1 in vivo, for treatment of cancer, and for inhibiting the onset of cancer.该披露涉及雷克西酮,包括式(I)和(II)的化合物或其药用可接受的盐、多型体、前药、溶剂合物或包合物。这些雷克西酮对于体内增加PD-L1,在癌症治疗中以及抑制癌症发生方面是有用的。
-
Improved and General Manganese‐Catalyzed N‐Methylation of Aromatic Amines Using Methanol作者:Jacob Neumann、Saravanakumar Elangovan、Anke Spannenberg、Kathrin Junge、Matthias BellerDOI:10.1002/chem.201605218日期:2017.4.24A novel lutidine‐based manganese PNP‐pincer complex has been synthesized for the selective N‐methylation of aromatic amines with methanol. Using borrowing hydrogen methodology, a selection of differently functionalized aniline derivatives is selectively methylated in good yields.
-
Rotaxane or Pseudorotaxane? Effects of Small Structural Variations on the Deslipping Kinetics of Rotaxanes with Stopper Groups of Intermediate Size作者:Ansgar Affeld、Gosia M. Hübner、Christian Seel、Christoph A. SchalleyDOI:10.1002/1099-0690(200108)2001:15<2877::aid-ejoc2877>3.0.co;2-r日期:2001.8Secondly, stopper flexibility has an important influence on the deslipping rate. Thirdly, exchange of a carbonamide for a sulfonamide in the wheel significantly reduces the entropic costs of the deslipping, resulting in a pronounced deslipping rate enhancement. Fourthly, intramolecular hydrogen bonding within the wheel decelerates deslipping by a factor of more than 104.三个系列的轮烷已通过滑动合成(轴和轮混合熔化)、通过识别大环轮内部的酰胺基团或通过阴离子模板法(其中塞子酚盐与轮以氢键键合)的方式合成。然后通过与半轴的反应连接。大多数这些轮烷使用的 3,5-二叔丁基苯基塞子足够大,可以隔离它们,但在适当的条件下仍允许车轮从轴上滑落。所有轮烷的脱滑活化参数均来自 1 H NMR 动力学测量值,并已根据 Arrhenius 方程和 Eyring 理论进行评估。小的结构变化会对激活参数产生惊人的影响。首先,在一些例子中,轴长影响防滑屏障,但止动器和车轮的尺寸互补性保持不变。其次,塞子的柔韧性对脱滑率有重要影响。第三,在车轮中用碳酰胺交换磺酰胺显着降低了脱滑的熵成本,从而显着提高了脱滑率。第四,车轮内的分子内氢键使滑移减速超过 104 倍。
表征谱图
-
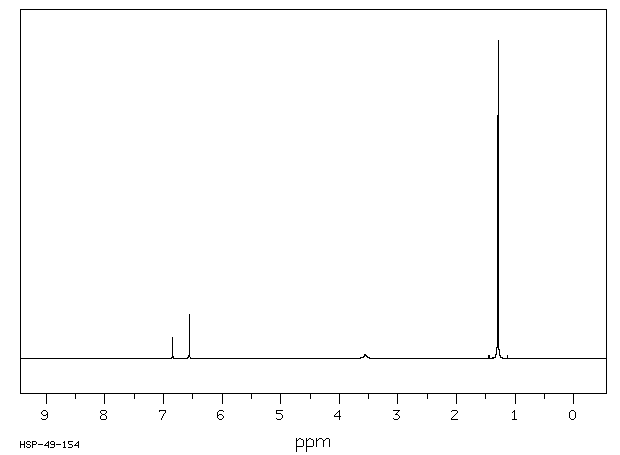
氢谱1HNMR
-
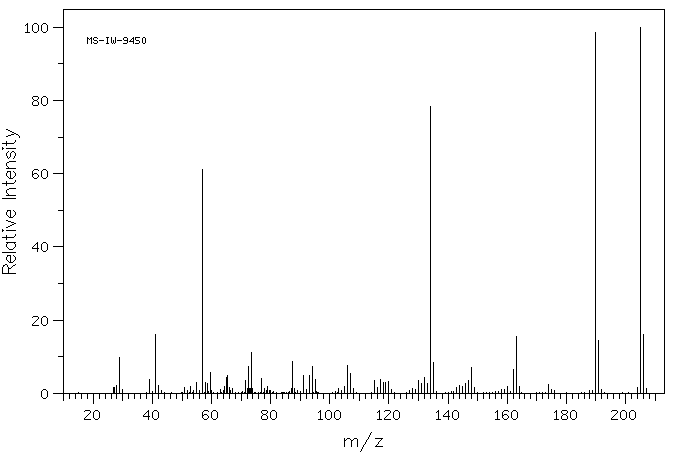
质谱MS
-
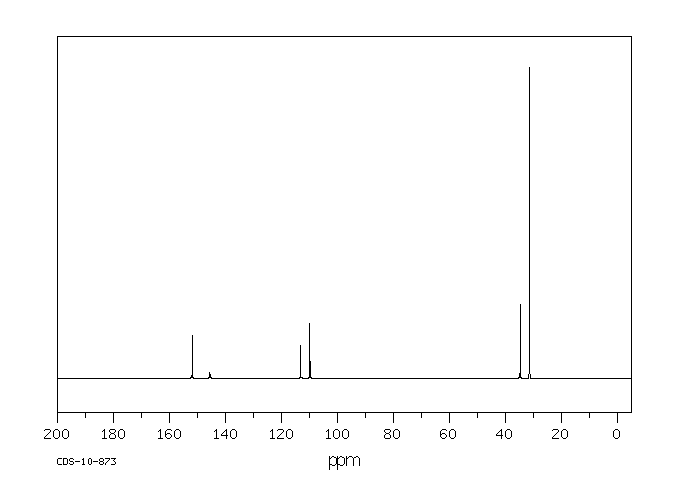
碳谱13CNMR
-
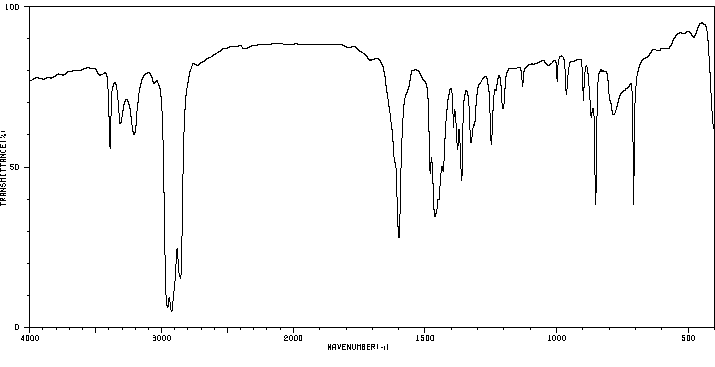
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫