3-(2-氟苯基)丙-2-烯酸 | 18944-77-9
物质功能分类
中文名称
3-(2-氟苯基)丙-2-烯酸
中文别名
2-氟邻氨基苯甲酸
英文名称
2-fluorocinnamic acid
英文别名
(E)-3-(2-fluorophenyl)acrylic acid;2-fluoro-trans-cinnamic acid;3-(2-fluoro-phenyl)-acrylic acid;2'-fluoro-(E)-cinnamic acid;o-fluorocinnamic acid;(E)-3-(2-fluorophenyl)prop-2-enoic acid
CAS
18944-77-9
化学式
C9H7FO2
mdl
MFCD00004370
分子量
166.152
InChiKey
IOUDZAFBPDDAMK-AATRIKPKSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:169.0-183.0 °C
-
沸点:281.6±15.0 °C(Predicted)
-
密度:1.285±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:存放在室温、干燥且密封的环境中。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
2-Fluorocinnamic acid
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
Ingredient name: 2-Fluorocinnamic acid
CAS number: 18944-77-9
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
This product should be handled only by, or under the close supervision of, those properly qualified
Handling:
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C9H7FO2
Molecular weight: 166.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen fluoride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
2-Fluorocinnamic acid
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
Ingredient name: 2-Fluorocinnamic acid
CAS number: 18944-77-9
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
This product should be handled only by, or under the close supervision of, those properly qualified
Handling:
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C9H7FO2
Molecular weight: 166.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen fluoride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— ethyl (E)-3-(2-fluorophenyl)acrylate 89760-42-9 C11H11FO2 194.206 邻氟肉桂醛 (E)-3-(2-fluorophenyl)acrylaldehyde 149733-71-1 C9H7FO 150.152 2-氟苯乙烯 2-fluorostyrene 394-46-7 C8H7F 122.142 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (E)-3-(2-氟苯基)丙烯酸甲酯 (E)-methyl 3-(2-fluorophenyl)acrylate 104201-65-2 C10H9FO2 180.179 —— ethyl (E)-3-(2-fluorophenyl)acrylate 89760-42-9 C11H11FO2 194.206 3-(2-氟苯基)丙-2-烯-1-醇 (E)-3-(2-fluorophenyl)prop-2-en-1-ol 807369-87-5 C9H9FO 152.168 —— (E)-t-butyl 3-(2-fluorophenyl)acrylate 444108-95-6 C13H15FO2 222.259 —— (E)-3-(2-fluoro-4-hydroxyphenyl)acrylic acid —— C9H7FO3 182.151 —— 2-fluorocinnamyl chloride 120681-05-2 C9H6ClFO 184.597 —— o-fluoro-cinnamyl methyl carbonate 918309-62-3 C11H11FO3 210.205 (E)-3-(2-氟苯基)丙-2-烯腈 (E)-3-(2'-fluorophenyl)-2-propenenitrile 91319-60-7 C9H6FN 147.152 —— (E)-3-(2-fluorophenyl)-N-isopropylacrylamide —— C12H14FNO 207.248 2-氟苯乙烯 2-fluorostyrene 394-46-7 C8H7F 122.142 —— (E)-2-(3-(2-fluorophenyl)acrylamido)acetic acid 1020656-52-3 C11H10FNO3 223.204 (E)-2-(3-(2-氟苯基)丙烯酰胺基)乙酸甲酯 (E)-2-(3-(2-fluorophenyl)acrylamido)acetic acid methyl ester 1020656-50-1 C12H12FNO3 237.231 —— (E)-3-(2-fluorophenyl)-N-methoxy-N-methylacrylamide 372183-83-0 C11H12FNO2 209.22 —— (E)-1-(2-bromovinyl)-2-fluorobenzene —— C8H6BrF 201.038 —— (E)-1-(2-cyclohexylvinyl)-2-fluorobenzene —— C14H17F 204.287 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:苯甲酸系烷基锂的生成和环化:4-取代茚满的制备。摘要:DOI:10.1021/jo972065z
-
作为产物:描述:2-氟苯甲醛 在 sodium hydride 、 sodium hydroxide 作用下, 以 四氢呋喃 、 乙醇 、 水 、 mineral oil 为溶剂, 生成 3-(2-氟苯基)丙-2-烯酸参考文献:名称:Synthesis of substituted nitroolefins: a copper catalyzed nitrodecarboxylation of unsaturated carboxylic acids摘要:使用催化量的CuCl (10 mol%)和特丁基硝酸酯(2 equiv.)作为硝化剂,在空气存在的条件下,实现了一种新颖、温和且便捷的取代肉桂酸衍生物的亚硝基脱羧反应,转化为相应的硝基烯烃。这一反应提供了一种合成β,β-二取代硝基烯烃衍生物的有效方法,这些化合物通常难以通过其他传统方法获得。此外,这一反应具有选择性,酸衍生物的E-异构体能够生成对应的E-硝基烯烃。该方法的另一个显著特点是,与其他方法不同,转化过程中无需使用金属硝酸盐或HNO3。DOI:10.1039/c3ob41408f
文献信息
-
Synthesis of Aryl Fluorides on a Solid Support and in Solution by Utilizing a Fluorinated Solvent作者:Marion Döbele、Sylvia Vanderheiden、Nicole Jung、Stefan BräseDOI:10.1002/anie.201001507日期:2010.8.9F for fast: The perfluorinated solvent C6F14 is the key to a new variant of the Balz–Schiemann reaction for the synthesis of fluorinated arenes. Triazenes are converted into fluoroarenes under mild conditions on a support and in solution (see scheme). The method is straightforward and inexpensive, and yields previously difficult‐to‐prepare fluoroarenes in high purity.
-
A novel phenylalanine ammonia-lyase from Pseudozyma antarctica for stereoselective biotransformations of unnatural amino acids作者:Andrea Varga、Pál Csuka、Orlavanah Sonesouphap、Gergely Bánóczi、Monica Ioana Toşa、Gabriel Katona、Zsófia Molnár、László Csaba Bencze、László Poppe、Csaba PaizsDOI:10.1016/j.cattod.2020.04.002日期:2021.4derivatives (rac-1a-s) by enzymatic ammonia elimination and also in the enantiotope selective ammonia addition reactions to cinnamic acid derivatives (2a-s). The enantiotope selectivity of PzaPAL with o-, m-, p-fluoro-, o-, p-chloro- and o-, m-bromo-substituted cinnamic acids proved to be higher than that of PcPAL.通过针对已知的PAL序列筛选微生物基因组,鉴定了一种新型的嗜冷酵母南极假单胞菌(PZa PAL)苯丙氨酸解氨酶。与真核生物的已知PAL相比,PZa PAL具有显着不同的底物结合口袋,带有一个延伸的环(长26 aa)连接到活性位点的芳香环结合区域。表征了在大肠杆菌中表达的重组PZa PAL的一般性质,包括该新PAL与1-苯丙氨酸(S)-1a和其他外消旋取代苯丙氨酸rac - 1b-g,k的动力学特征。。在大多数情况下,PZa PAL的营业额明显高于Petroselinum crispum(Pc PAL)的PAL。最后,在外消旋苯丙氨酸衍生物(rac - 1a-s)通过酶促氨消除的动力学拆分以及对映体选择性氨加成肉桂酸衍生物(2a-s)的动力学拆分中,比较了PZa PAL和Pc PAL的生物催化性能。。的enantiotope选择性PZA PAL与ö - ,米- ,p -氟,ø - ,p氯代和ø
-
[EN] PHENYLALKYL SULFAMATE COMPOUND AND MUSCLE RELAXANT COMPOSITION COMPRISING THE SAME<br/>[FR] COMPOSÉ DE SULFAMATE DE PHÉNYLALKYLE ET COMPOSITION MYORELAXANTE LE CONTENANT申请人:BIO PHARM SOLUTIONS CO LTD公开号:WO2013187727A1公开(公告)日:2013-12-19The present invention relates to novel phenylalkyl sulfamate compounds, a method for preventing or treating a disease associated with muscle spasm. The present invention ensures the enhancement of muscle relaxation activity essential for alleviation of muscle spasm, such that it is promising for preventing or treating various diseases associated with muscle spasm.本发明涉及新型苯基烷基磺酰胺化合物,一种用于预防或治疗与肌肉痉挛相关疾病的方法。本发明确保增强肌肉松弛活性,这对于缓解肌肉痉挛至关重要,因此有望预防或治疗与肌肉痉挛相关的各种疾病。
-
N-substituted nonaryl-heterocyclic NMDA/NR2B antagonists
-
Synthesis and antitumor activity of 1,3,4-oxadiazole possessing 1,4-benzodioxan moiety as a novel class of potent methionine aminopeptidase type II inhibitors作者:Juan Sun、Ming-Hui Li、Shao-Song Qian、Feng-Jiao Guo、Xiao-Fang Dang、Xiao-Ming Wang、Ya-Rong Xue、Hai-Liang ZhuDOI:10.1016/j.bmcl.2013.03.068日期:2013.5A series of 1,3,4-oxadiazole derivatives containing 1,4-benzodioxan moiety (7a–7q) have been designed, synthesized and evaluated for their antitumor activity. Most of the synthesized compounds were proved to have potent antitumor activity and low toxicity. Among them, compound 7a showed the most potent biological activity against Human Umbilical Vein Endothelial cells, which was comparable to the positive
表征谱图
-
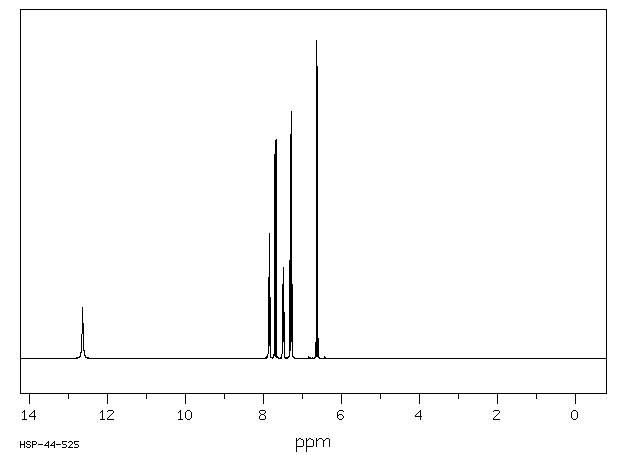
氢谱1HNMR
-
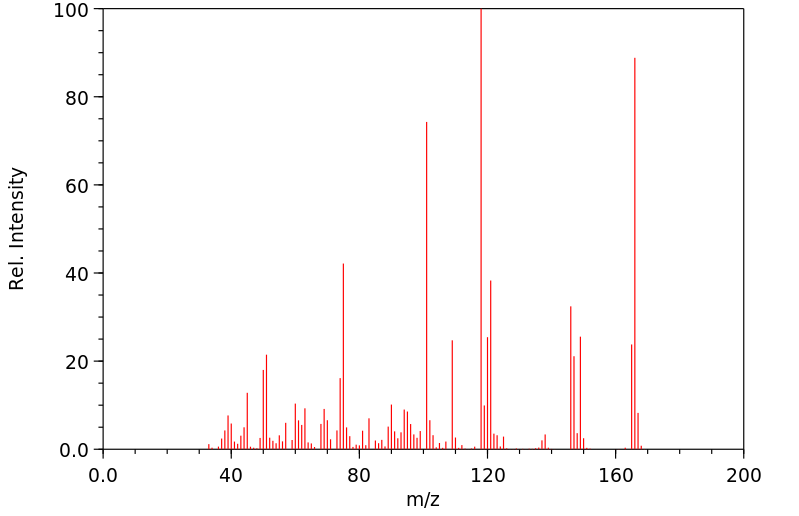
质谱MS
-
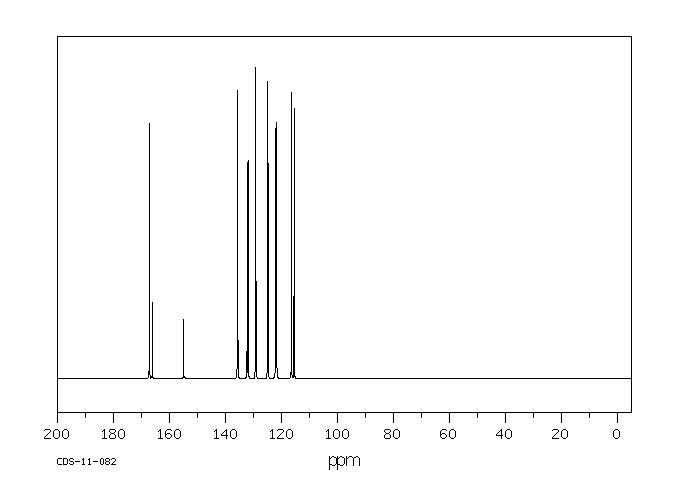
碳谱13CNMR
-
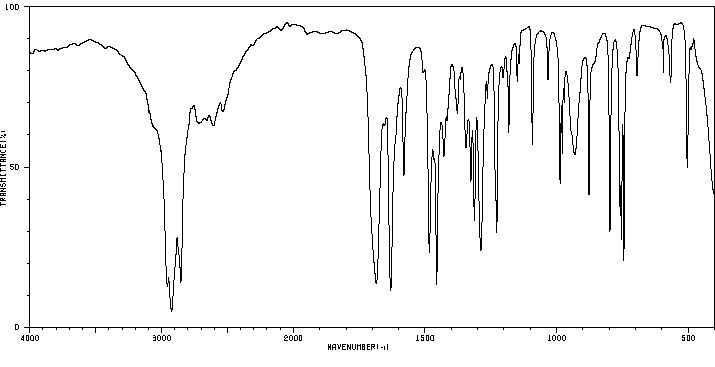
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E)-3-(4-(叔丁基)苯基)丙烯酸乙酯
(E)-3-(2-(三氟甲基)苯基)丙烯酸乙酯
(E)-3-(2,4-二甲氧基苯基)丙烯酸乙酯
(2E)-N-[2-(3-羟基-2-氧代-2,3-二氢-1H-吲哚-3-基)乙基]-3-苯基丙-2-烯酰胺
黄金树苷
鲁索曲波帕
香豆酸肉桂酯
香豆酰多巴胺
香草醛缩丙酮
顺式邻羟基肉桂酸
顺式芥子酸
顺式-曲尼司特
顺式-乙基肉桂酸酯
顺式-N-阿魏酰酪胺
顺式-3,4-二甲氧基苯丙烯酸
顺式-2-((叔丁氧羰基)氨基)-3-(4-氨甲酰基-2,6-二甲苯基)丙烯酸甲酯
顺-o-羧基肉桂酸
顺-2-甲氧基肉桂酸
阿魏酸钠
阿魏酸酰胺
阿魏酸甲酯
阿魏酸甲酯
阿魏酸甲酯
阿魏酸松柏酯
阿魏酸杂质1
阿魏酸异辛酯
阿魏酸哌嗪
阿魏酸二十烷基酯
阿魏酸乙酯
阿魏酸4-O-硫酸二钠盐
阿魏酸-D3
阿魏酸
阿魏酸
阿魏酰酪胺
间羟基肉桂酸
间羟基肉桂酸
间硝基肉桂酸
间甲基肉桂酸
间甲基反式肉桂酸甲酯
间氯肉桂酸
间三氟甲氧基肉桂酸甲酯
间-香豆酸
间-(三氟甲基)-肉桂酸
锂(E)-2-溴-3-苯基丙烯酸酯
钠二乙基2-[(氧代氨基)-苯基亚甲基]丙二酸酯盐
酪氨酸磷酸化抑制剂AG 556
酪氨酸磷酸化抑制剂AG 527
酪氨酸磷酸化抑制剂AG 490
酪氨酸磷酸化抑制剂A46
酪氨酸磷酸化抑制剂 AG 30