邻甲氧基-2-溴苯乙酮 | 31949-21-0
中文名称
邻甲氧基-2-溴苯乙酮
中文别名
2-溴-2'-甲氧基苯乙酮;Alpha-溴-2’-甲氧基苯乙酮
英文名称
bromomethyl 2-methoxyphenyl ketone
英文别名
2-Bromo-2'-methoxyacetophenone;2-bromo-1-(2-methoxyphenyl)ethan-1-one;2-bromo-1-(2-methoxyphenyl)ethanone;2-bromo-2′-methoxyacetophenone
CAS
31949-21-0
化学式
C9H9BrO2
mdl
MFCD00000196
分子量
229.073
InChiKey
GKNCPTLOPRDYMH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:43-45 °C(lit.)
-
沸点:130 °C1 mm Hg(lit.)
-
密度:1.4921 (rough estimate)
-
闪点:>230 °F
-
稳定性/保质期:
如果遵照规格使用和储存,则不会分解,未有已知危险反应。避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:8
-
危险品标志:T,C
-
安全说明:S22,S26,S36/37/39,S45
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2914700090
-
危险品运输编号:UN 3261 8/PG 2
-
包装等级:III
-
危险类别:8
-
危险性防范说明:P301+P330+P331,P303+P361+P353,P363,P304+P340,P310,P321,P260,P264,P280,P305+P351+P338,P405,P501
-
危险性描述:H314
-
储存条件:密封保存,并冷藏以保证新鲜度。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
2-Bromo-2’-methoxyacetophenone
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H314: Causes severe skin burns and eye damage
P280: Wear protective gloves/protective clothing/eye protection/face protection
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P310: Immediately call a POISON CENTER or doctor/physician
Section 3. Composition/information on ingredients.
Ingredient name: 2-Bromo-2’-methoxyacetophenone
CAS number: 31949-21-0
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
This product should be handled only by, or under the close supervision of, those properly qualified
Handling:
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C9H9BrO2
Molecular weight: 229.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
UN Number: UN3261 Class: 8 Packing group: II
Proper shipping name: CORROSIVE SOLID, ACIDIC, ORGANIC, N.O.S. (2-Bromo-2’-methoxyacetophenone)
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
2-Bromo-2’-methoxyacetophenone
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H314: Causes severe skin burns and eye damage
P280: Wear protective gloves/protective clothing/eye protection/face protection
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P310: Immediately call a POISON CENTER or doctor/physician
Section 3. Composition/information on ingredients.
Ingredient name: 2-Bromo-2’-methoxyacetophenone
CAS number: 31949-21-0
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
This product should be handled only by, or under the close supervision of, those properly qualified
Handling:
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C9H9BrO2
Molecular weight: 229.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
UN Number: UN3261 Class: 8 Packing group: II
Proper shipping name: CORROSIVE SOLID, ACIDIC, ORGANIC, N.O.S. (2-Bromo-2’-methoxyacetophenone)
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— α,α-dibromo-2'-methoxyacetophenone 1121543-12-1 C9H8Br2O2 307.969 2'-甲氧基苯乙酮 2-Methoxyacetophenone 579-74-8 C9H10O2 150.177 2'-羟基苯乙酮 o-hydroxyacetophenone 118-93-4 C8H8O2 136.15 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2'-甲氧基苯乙酮 2-Methoxyacetophenone 579-74-8 C9H10O2 150.177 2-溴-1-(5-溴-2-甲氧基苯基)乙酮 2-bromo-1-(5-bromo-2-methoxyphenyl)ethanone 67639-58-1 C9H8Br2O2 307.969 —— 2-amino-1-(2-methoxyphenyl)ethan-1-one 189506-45-4 C9H11NO2 165.192 —— 1-(2-methoxyphenyl)prop-2-en-1-one 77942-10-0 C10H10O2 162.188 2-羟基-2-甲氧基苯乙酮 2-hydroxy-1-(2-methoxyphenyl)ethanone 224321-19-1 C9H10O3 166.177 2-甲氧基苯乙基溴 1-(2-bromoethyl)-2-methoxybenzene 36449-75-9 C9H11BrO 215.09 2-甲氧基苯甲酰乙腈 3-(2-methoxyphenyl)-3-oxopropanenitrile 35276-83-6 C10H9NO2 175.187 —— (E)-1-(2-methoxyphenyl)but-2-en-1-one 40872-81-9 C11H12O2 176.215 —— 1-(2-methoxy-phenyl)-2-methylamino-ethanone —— C10H13NO2 179.219 1-(2-甲氧基苯基)-2-苯乙酮 1-(2-methoxyphenyl)-2-phenylethanone 33470-10-9 C15H14O2 226.275 2-叠氮基-1-(2-甲氧基苯基)-乙酮 2-azido-1-(2-methoxyphenyl)ethan-1-one 34635-38-6 C9H9N3O2 191.189 —— 1,4-bis(2-methoxyphenyl)butane-1,4-dione 134179-55-8 C18H18O4 298.339 —— 3,3,3-trifluoro-1-(2-methoxyphenyl)propan-1-one 130654-91-0 C10H9F3O2 218.175 1-(2-甲氧基苯基)-3-苯基-丙烷-1,3-二酮 1-(2-methoxyphenyl)-3-phenylpropane-1,3-dione 41126-22-1 C16H14O3 254.285 —— 2-(2-methoxyphenyl)-2-oxoethyl acetate 74786-55-3 C11H12O4 208.214 —— 2-(Diformyl-amino)-2'-methoxyacetophenone 473693-43-5 C11H11NO4 221.213 —— 1-(2-methoxybenzoyl)-2-(4-methoxybenzoyl)ethane 134179-54-7 C18H18O4 298.339 —— S-[2-(2-methoxyphenyl)-2-oxoethyl] ethanethioate 1121543-13-2 C11H12O3S 224.28 —— 1-(2-methoxyphenyl)-2-(2,2,2-trifluoroethoxy)ethanone —— C11H11F3O3 248.202 —— ω-Phenoxy-2-methoxy-acetophenon 28512-96-1 C15H14O3 242.274 —— 1-(2-Methoxyphenyl)-2-(piperazin-1-yl)ethan-1-one 1021901-99-4 C13H18N2O2 234.298 —— 2-Ethylsulfanylcarbothioylsulfanyl-1-(2-methoxyphenyl)ethanone 948837-75-0 C12H14O2S3 286.44 —— 2-(3-Methoxyphenoxy)-1-(2-methoxyphenyl)ethanone 1086085-26-8 C16H16O4 272.301 —— 2-bromo-1-(2-methoxyphenyl)ethan-1-ol 142363-61-9 C9H11BrO2 231.089 —— 1-(2-methoxyphenyl)-2-[(4-methoxyphenyl)amino]ethan-1-one 1268077-17-3 C16H17NO3 271.316 —— 2-(o-methoxyphenacyl)cyclohexanone 54669-81-7 C15H18O3 246.306 —— dimethyl [2-(2-methoxyphenyl)-2-oxoethyl]phosphonate 918874-30-3 C11H15O5P 258.211 —— 2-((4H-1,2,4-triazol-3-yl)thio)-1-(2-methoxyphenyl)ethanone —— C11H11N3O2S 249.293 - 1
- 2
- 3
反应信息
-
作为反应物:描述:邻甲氧基-2-溴苯乙酮 在 palladium on activated charcoal palladium on activated charcoal 、 N-溴代丁二酰亚胺(NBS) 、 氢气 、 三乙胺 作用下, 以 四氢呋喃 、 甲醇 为溶剂, 反应 7.0h, 生成 4-(2-甲氧基苯基)-2-[(5-甲基-1H-咪唑-4-基)甲基]-1,3-噻唑参考文献:名称:Synthesis, in vitro binding profile, and CNS penetrability of the highly potent 5-HT3 receptor antagonist [3H]-4-(2-methoxyphenyl)-2-[4(5)-methyl-5(4)-imidazolylmethyl]thiazole摘要:4-(2-Methoxyphenyl)-2-[4(5)-methyl-5(4)-imidazolylmethyl]thiazole (5) is a highly potent member of a structurally novel series of selective serotonin-3 receptor antagonists. The synthesis of tritiated 5 and its binding profile in neuroblastoma-glioma 108-15 cells are described. Furthermore, in vivo studies in rat with this radioligand indicate that it effectively penetrates the blood-brain barrier upon peripheral administration. Thus, 5 should be a useful pharmacological tool for both in vitro and in vivo studies of this class of compounds.DOI:10.1021/jm00173a017
-
作为产物:描述:2'-羟基苯乙酮 在 potassium hydroxide 、 copper(ll) bromide 作用下, 以 氯仿 、 二甲基亚砜 、 乙酸乙酯 为溶剂, 反应 0.33h, 生成 邻甲氧基-2-溴苯乙酮参考文献:名称:使用串联交叉复分解/分子内氢化芳基化序列的功能化四氢咔唑的高效途径摘要:描述了新型钌催化的串联交叉复分解/分子内氢芳基化序列的范围。这种方法可以有效,高效地合成结构多样且复杂的四氢咔唑(高达98%)。而且,提出了通过用钌配合物和手性胺的顺序催化来开发当前方法的对映选择性形式的初步努力,其具有高收率和对映选择性(高达88%的收率和91%的ee)。DOI:10.1002/asia.201000315
-
作为试剂:参考文献:名称:Derivatized alkanolamines as cardiovascular agents摘要:以下结构式##STR1##的新型衍生烷醇胺被描述为有用的心血管药物。特别描述了它们作为表现出抗心律失常作用的心血管药物的有用性。所述的抗心律失常作用属于II类/III类结合型。还描述了含有这类化合物的药物配方。公开号:US05051423A1
文献信息
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
[EN] PROCESSES FOR THE PREPARATION OF FUNGICIDAL COMPOUNDS<br/>[FR] PROCÉDÉS DE PRÉPARATION DE COMPOSÉS FONGICIDES申请人:GILEAD APOLLO LLC公开号:WO2018161008A1公开(公告)日:2018-09-07Provided herein are processes for the preparation of stereomerically enriched compounds of Formulas I-014, I-020, I-064, I-074, I-082, I-089, I-090, I-095, I-171, I-181, I-184, I-186, I-189, I-191, I-192, I-193, I-205, I-206, I-208, I-211, I-212, I-213, I-220, I-229, I-231, I-233, I-234, I-246, I-251, I-258, I-259, I-262, I-263, I-285, I-323 and I-400. The compounds described herein exhibit activity as pesticides and are useful, for example, in methods for the control of fungal pathogens and diseases caused by fungal pathogens in plants. A preferred process is directed to preparing a stereomerically enriched compound of Formula V-1 or V-2-F by assymetrical reduction in the presence of a chiral organometallic catalyst.本文提供了制备具有I-014、I-020、I-064、I-074、I-082、I-089、I-090、I-095、I-171、I-181、I-184、I-186、I-189、I-191、I-192、I-193、I-205、I-206、I-208、I-211、I-212、I-213、I-220、I-229、I-231、I-233、I-234、I-246、I-251、I-258、I-259、I-262、I-263、I-285、I-323和I-400的立体富集化合物的方法。本文描述的化合物表现出作为杀虫剂的活性,并且在例如用于控制植物中由真菌病原体引起的真菌病害的方法中是有用的。一种首选的方法是通过在手性有机金属催化剂存在下进行不对称还原来制备具有V-1或V-2-F式的立体富集化合物。
-
TRIAZOLE ACC INHIBITORS AND USES THEREOF
-
Microwave-Assisted Preparation of Fused Bicyclic Heteroaryl Boronates: Application in One-Pot Suzuki Couplings作者:Erin F. DiMauro、Jason R. VitulloDOI:10.1021/jo060218p日期:2006.5.1The rapid and efficient synthesis of various disubstituted 5,6-fused heterocycles using a microwave-assisted one-pot cyclization−Suzuki coupling approach is described. This work highlights the tolerance of the boronic ester functional group to a variety of reaction conditions and the utility of functionalized boronates as penultimate intermediates in the synthesis of diverse compound libraries.
-
Metal-Free Regioselective Alkylation of Imidazo[1,2-a]pyridines with N-Hydroxyphthalimide Esters under Organic Photoredox Catalysis作者:Can Jin、Bin Sun、Tengwei Xu、Liang Zhang、Rui Zhu、Jin Yang、Min XuDOI:10.1055/s-0039-1691567日期:2020.3A visible-light-induced direct C–H alkylation of imidazo[1,2-a]pyridines has been developed. It proceeds at room temperature by employing inexpensive Eosin Y as a photocatalyst and alkyl N-hydroxyphthalimide (NHP) esters as alkylation reagents. A variety of NHP esters derived from aliphatic carboxylic acids (primary, secondary, and tertiary) were tolerated in this protocol, giving the corresponding
表征谱图
-
氢谱1HNMR
-
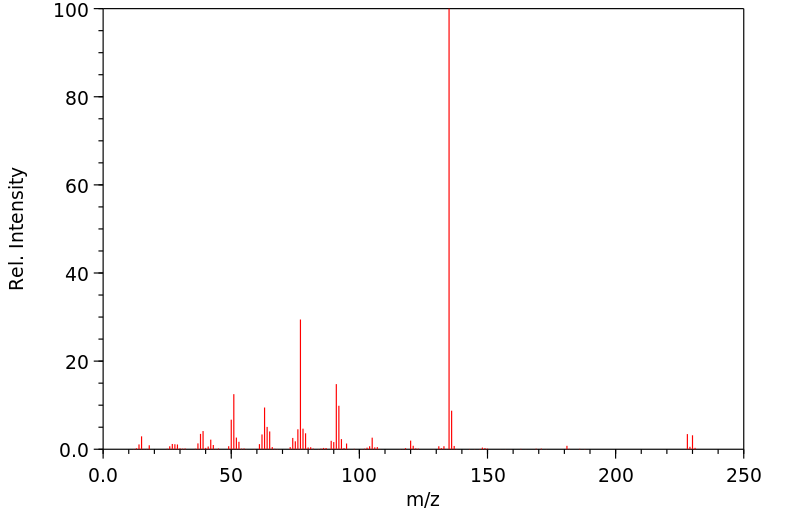
质谱MS
-
碳谱13CNMR
-
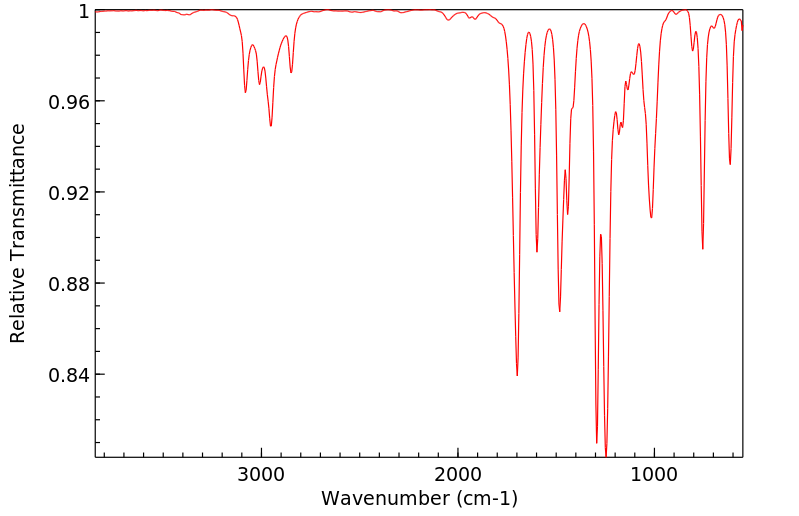
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷