六甘醇 | 2615-15-8
中文名称
六甘醇
中文别名
六聚乙二醇;六乙烯二醇;六乙二醇;硫乙烯基乙二醇
英文名称
Hexaethylene glycol
英文别名
3,6,9,12,15-pentaoxaheptadecane-1,17-diol;2-[2-[2-[2-[2-(2-hydroxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethanol
CAS
2615-15-8
化学式
C12H26O7
mdl
MFCD00002877
分子量
282.334
InChiKey
IIRDTKBZINWQAW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:5-7 °C (lit.)
-
沸点:217 °C/4 mmHg (lit.)
-
密度:1.127 g/mL at 25 °C (lit.)
-
闪点:>110°C
-
溶解度:与氯仿和甲醇混溶。
-
保留指数:2081.8;2078
-
稳定性/保质期:
如果按照规格正确使用和储存,则不会发生分解。
计算性质
-
辛醇/水分配系数(LogP):-1.9
-
重原子数:19
-
可旋转键数:16
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:86.6
-
氢给体数:2
-
氢受体数:7
安全信息
-
安全说明:S23,S24/25
-
WGK Germany:2
-
危险品运输编号:NONH for all modes of transport
-
海关编码:2909499000
-
RTECS号:MM3670000
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:密封于阴凉干燥处。
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : Hexaethylene glycol
CAS-No. : 2615-15-8
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Not a hazardous substance or mixture according to Regulation (EC) No 1272/2008
This substance is not classified as dangerous according to Directive 67/548/EEC.
Label elements
Labelling according Regulation (EC) No 1272/2008 [CLP]
Pictogram none
Signal word none
Hazard statement(s) none
Precautionary statement(s) none
Supplemental Hazard none
Statements
The following percentage of the mixture consists of ingredient(s) with unknown acute toxicity: 100 %
The following percentage of the mixture consists of ingredient(s) with unknown hazards to the aquatic
environment: 100 %
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Synonyms : 3,6,9,12,15-Pentaoxaheptadecane-1,17-diol
Formula : C12H26O7
Molecular Weight : 282,33 g/mol
Section 4. FIRST AID MEASURES
Description of first aid measures
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration.
In case of skin contact
Wash off with soap and plenty of water.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water.
Most important symptoms and effects, both acute and delayed
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Avoid breathing vapors, mist or gas.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Keep in suitable, closed containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Normal measures for preventive fire protection.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are
opened must be carefully resealed and kept upright to prevent leakage.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
General industrial hygiene practice.
Personal protective equipment
Eye/face protection
Use equipment for eye protection tested and approved under appropriate government standards
such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
impervious clothing, The type of protective equipment must be selected according to the
concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Respiratory protection not required. For nuisance exposures use type OV/AG (US) or type ABEK
(EU EN 14387) respirator cartridges. Use respirators and components tested and approved under
appropriate government standards such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: liquid, clear, viscous
Colour: light yellow
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing Melting point/range: 5 - 7 °C - lit.
point
f) Initial boiling point and 217 °C at 5 hPa - lit.
boiling range
g) Flash point > 113,00 °C - closed cup
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density 1,127 g/mL at 25 °C
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
LD50 Oral - rat - 32.000 mg/kg
Remarks: Behavioral:Somnolence (general depressed activity). Gastrointestinal:Other changes. Kidney,
Ureter, Bladder:Other changes.
Skin corrosion/irritation
Serious eye damage/eye irritation
Eyes - rabbit - Mild eye irritation
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
Specific target organ toxicity - single exposure
Specific target organ toxicity - repeated exposure
Aspiration hazard
Potential health effects
Inhalation May be harmful if inhaled. May cause respiratory tract irritation.
Ingestion May be harmful if swallowed.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes May cause eye irritation.
Additional Information
RTECS: MM3670000
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
Mobility in soil
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
Section 15. REGULATORY INFORMATION
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
no data available
Section 16. OTHER INFORMATION
Further information
Copyright 2012 Co. LLC. License granted to make unlimited paper copies for internal use
only.
The above information is believed to be correct but does not purport to be all inclusive and shall be
used only as a guide. The information in this document is based on the present state of our knowledge
and is applicable to the product with regard to appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Corporation and its Affiliates shall not be held
liable for any damage resulting from handling or from contact with the above product. See
and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.
制备方法与用途
化学反应
六甘醇由于分子结构中的氧原子存在,使其电负性大于丙酸,更易于发生化学反应。作为一种活跃的有机化学中间体,在精细化工领域有着重要的应用。
应用六甘醇主要用于生产农药、染料、农林化学品和医药中间体等,与人们的日常生活密切相关。
合成将36.6 g(45.0 mmol)的庚乙二醇二硬脂酸酯、150 mL的CH2Cl2和0.677 g的10%钯/碳装入带有玻璃插件的高压蒙乃尔合金弹中。在室温下以50大气压的H2进行氢解48小时后,过滤器-催化剂(可重复使用),并用 洗涤滤液。浓缩滤液得到三苯基甲烷和油的混合物。将此混合物溶解于沸腾的甲醇中,在冷却至0°C时使大部分三苯基甲烷结晶析出,然后进行过滤,并用己烷洗涤六次以去除痕量的三苯基甲烷,最终得到六甘醇。

用途上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 三乙二醇 2,2'-[1,2-ethanediylbis(oxy)]bisethanol 112-27-6 C6H14O4 150.175 三缩四乙二醇 Tetraethylene glycol 112-60-7 C8H18O5 194.228 二乙二醇 diethylene glycol 111-46-6 C4H10O3 106.122 —— α-allyl-ω-allyloxytetra(oxyethylene) 58185-54-9 C14H26O5 274.357 双[2-(2-氯化乙氧基)乙基]醚 1,11-dichloro-3,6,9-trioxaundecane 638-56-2 C8H16Cl2O3 231.119 2-[2-[2-[2-[2-(2-氯乙氧基)乙氧基]乙氧基]乙氧基]乙氧基]乙醇 17-chloro-3,6,9,12,15-pentaoxaheptadecan-1-ol 28746-78-3 C12H25ClO6 300.78 溴代-六聚乙二醇 17-bromo-3,6,9,12,15-pentaoxaheptadecan-1-ol 136399-05-8 C12H25BrO6 345.231 3,6,9,12,15-五氧杂十七烷-1,17-二基二-溴化物 1,17-dibromo-3,6,9,12,15-pentaoxaheptadecane 67705-77-5 C12H24Br2O5 408.128 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 六乙二醇单甲醚 hexaethylene glycol monomethyl ether 23601-40-3 C13H28O7 296.361 聚乙二醇单甲醚 2,5,8,11,14,17,20,23,26-Nonaoxaoctacosan-28-ol 6048-68-6 C19H40O10 428.521 七甘醇单甲醚 3,6,9,12,15,18,21-heptaoxadocosan-1-ol 4437-01-8 C15H32O8 340.414 [60]冠20 <60>crown-20 71092-63-2 C40H80O20 881.063 —— 30-crown-10 52985-64-5 C20H40O10 440.532 18-冠醚-6 18-crown-6 ether 17455-13-9 C12H24O6 264.319 十甘醇 decaethylene glycol 5579-66-8 C20H42O11 458.547 三缩四乙二醇 Tetraethylene glycol 112-60-7 C8H18O5 194.228 羟丙基淀粉磷酸酯 Pentaethylene glycol 4792-15-8 C10H22O6 238.281 三乙二醇 2,2'-[1,2-ethanediylbis(oxy)]bisethanol 112-27-6 C6H14O4 150.175 九甘醇 nonaethylene glycol 3386-18-3 C18H38O10 414.494 十四甘醇 tetradecaethylene glycol 67411-64-7 C28H58O15 634.76 —— <36>crown-12 71092-59-6 C24H48O12 528.638 —— 54-Crown-18 71092-62-1 C36H72O18 792.957 十八乙二醇 PEG1000 4445-03-8 C36H74O19 810.972 —— 33-Crown-11 52985-65-6 C22H44O11 484.585 —— hexaethylene glycol diethyl ether 4435-24-9 C16H34O7 338.442 八乙二醇 octaethylene glycol 5117-19-1 C16H34O9 370.441 —— 3,6,9,12,15,18-hexaoxaeicosan-1-ol 4403-58-1 C14H30O7 310.388 二乙二醇 diethylene glycol 111-46-6 C4H10O3 106.122 —— 2-(2-(2-(2-(2-(2-allyloxyethoxy)ethoxy)ethoxy)ethoxy)ethoxy)ethan-1-ol 87048-16-6 C15H30O7 322.399 —— α-allyl-ω-allyloxyhexa(oxyethylene) 332941-23-8 C18H34O7 362.464 —— 1-propene hexaethylene glycol monomethyl ether 150669-65-1 C16H32O7 336.426 丙炔基-六聚乙二醇 3,6,9,12,15,18-hexaoxahenicos-20-yn-1-ol 944560-99-0 C15H28O7 320.383 2-[2-[2-[2-[2-(2-羟基乙氧基)乙氧基]乙氧基]乙氧基]乙氧基]乙醇,二氯化 1,17-dichloro-3,6,9,12,15-pentaoxaheptadecane 52559-90-7 C12H24Cl2O5 319.226 溴代-六聚乙二醇 17-bromo-3,6,9,12,15-pentaoxaheptadecan-1-ol 136399-05-8 C12H25BrO6 345.231 2-{2-[2-(2-{2-[2-(2-{2-[2-(2-氨基-乙氧基)-乙氧基]-乙氧基}-乙氧基)-乙氧基]-乙氧基}-乙氧基)-乙氧基]-乙氧基}-乙基胺 2-(2-(2-(2-(2-(2-(2-(2-(2-(2-aminoethoxy)ethoxy)ethoxy)ethoxy)ethoxy)ethoxy)ethoxy)ethoxy)ethoxy)ethanamine 474082-35-4 C20H44N2O9 456.577 3,6,9,12,15-五氧杂十七烷-1,17-二基二-溴化物 1,17-dibromo-3,6,9,12,15-pentaoxaheptadecane 67705-77-5 C12H24Br2O5 408.128 17-氨基-3,6,9,12,15-五氧杂十七烷醇 3,6,9,12,15,18-hexaoxa-1-octadecyl amine 39160-70-8 C12H27NO6 281.349 丙炔基-六聚乙二醇-丙炔基 1,17-bis(2-propyn-1-oxy)-3,6,9,12,15-pentaoxaheptadecane 400775-35-1 C18H30O7 358.432 1,17-二氨基-3,6,9,12,15-五氧杂十七烷 1,17-diamino-3,6,9,12,15-pentaoxaheptadecane 72236-26-1 C12H28N2O5 280.365 3,6,9,12,15,18-六氧杂十九烷-1-胺 2,5,8,11,14,17-hexaoxanonadecan-19-amine 184357-46-8 C13H29NO6 295.376 甲基-六聚乙二醇-溴代 1-bromo-2-[2-(2-{2-[2-(2-methoxy-ethoxy)-ethoxy]-ethoxy}-ethoxy)-ethoxy]-ethane 125562-29-0 C13H27BrO6 359.258 氨基-十聚乙二醇 29-amino-3,6,9,12,15,18,21,24,27-nonaoxanonacosan-1-ol 129449-09-8 C20H43NO10 457.562 1-氨基-3,6,9-三噁-11-十一醇 11-amino-3,6,9-trioxaundecanol 86770-74-3 C8H19NO4 193.243 氨氧基-五聚乙二醇-氨氧基 3,6,9,12,15-penatoxa-heptadecane-1,17-dioxyamine 894414-82-5 C12H28N2O7 312.364 2-[2-[2-[2-[2-(2-氯乙氧基)乙氧基]乙氧基]乙氧基]乙氧基]乙醇 17-chloro-3,6,9,12,15-pentaoxaheptadecan-1-ol 28746-78-3 C12H25ClO6 300.78 3,6,9-三氧杂十一烷-1,11-二胺 2-[2-[2-[2-aminoethoxy]ethoxy]ethoxy]ethylamine 929-75-9 C8H20N2O3 192.258 2-[2-[2-[2-[2-[2-[2-(羧基甲氧基)乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙酸 3,6,9,12,15,18,21-heptaoxatricosane-1,23-dioic acid 83824-29-7 C16H30O11 398.408 3,6,9,12,15,18-六氧杂十九烷酸 2,5,8,11,14,17-hexaoxanonadecan-19-oic acid 16142-03-3 C13H26O8 310.345 —— (1-mercaptoprop-3-yl)hexa(ethylene glycol) 1426654-80-9 C15H32O7S 356.481 —— 4,7,10,13,16,19,22-heptaoxapentacosane-1,25-diamine 96146-49-5 C18H40N2O7 396.525 甲氧基-七聚乙二醇-乙酸 2,5,8,11,14,17,20-heptaoxadocosan-22-oic acid 75427-75-7 C15H30O9 354.398 炔基六聚乙二醇巯基 Propargyl-PEG6-SH 1422540-91-7 C15H28O6S 336.45 - 1
- 2
- 3
- 4
- 5
反应信息
-
作为反应物:参考文献:名称:Fast and efficient MCR-based synthesis of clickable rhodamine tags for protein profiling摘要:蛋白质分析探针是研究蛋白质组组成的重要工具,因此对了解高等生物体内各种复杂的生物过程做出了巨大贡献。为此,应用荧光标记的活性或亲和性探针是非常理想的。特别是在活体检测低含量目标蛋白时,荧光标记的分析探针具有很高的价值,否则很难通过标准印迹技术进行分析。本文介绍了一种基于 Ugi-4 组分反应(Ugi-4-CR)的一次性合成活化荧光标签(即叠氮、炔基或 NHS)的方法。由于形成了类胨结构,产物的荧光特性对 pH 值不敏感。此外,还将以标记模型蛋白质 BSA 为例,讨论这些探针的适用性。DOI:10.1039/c1ob06581e
-
作为产物:描述:hexaethylene glycol dibenzyl ether 在 palladium on activated charcoal 氢气 作用下, 以 盐酸 、 乙醇 为溶剂, 以91%的产率得到六甘醇参考文献:名称:苯并氮杂鎓和苯胺阳离子套索状醚配合物的合成和性质:“鸵鸟分子”配合物和分子内侧臂-大环相互作用的证据摘要:报道了几种新型套索状醚(具有带有侧链带有侧基供体基团的大环冠聚醚)的制备。这些化合物是衍生自已知的2-羟甲基-15-冠-5或-21-冠-7的醚。侧臂包括2-氨基苯基,2,4-二氨基苯基,2-硝基苯基,2-(3'-硝基联苯)和2-(3'-氨基联苯)。在一些情况下,氨基被转化为铵盐,该铵盐通过分子内氢键显示出基本的稳定性。同样,-NH 2 +。,BF 3 -该配合物显示出分子内氢键的证据。氨基联苯残基的重氮化反应产生了一个烯二唑鎓阳离子,该阳离子经历了分子内冠状络合,这是通过红外光谱研究判断形成的,我们称之为“鸵鸟分子”复合物。将N,N-二甲基苯胺添加到分子内的芳构氮鎓阳离子络合物中可制得偶氮化合物,但euro移试剂研究清楚地表明,重氮鎓阳离子在大环之外发生了反应。DOI:10.1016/0040-4020(84)85070-x
-
作为试剂:描述:参考文献:名称:HPLC-free in situ18F-fluoromethylation of bioactive molecules by azidation and MTBD scavenging摘要:使用叠氮和MTBD的顺序使用,生成纯[18F]氟甲基对甲苯磺酰基,并清除未反应的去甲基前体,为18F-氟甲基化合物的无HPLC合成提供了高效策略。DOI:10.1039/c9cc04901k
文献信息
-
Supramolecular gelation of alcohol and water by synthetic amphiphilic gallic acid derivatives作者:Hitoshi Tamiaki、Keishiro Ogawa、Keisuke Enomoto、Kazutaka Taki、Atsushi Hotta、Kazunori TomaDOI:10.1016/j.tet.2010.01.002日期:2010.2The supramolecular organogelation of alcohols was observed in relatively hydrophobic amphiphiles with a short oligo(ethylene glycol) unit and three long alkyl chains at room temperature, while the hydrogelation occurred in more hydrophilic gelators with a longer poly(ethylene glycol) unit and two long alkyl chains at various temperatures. When a hot aqueous solution of some of the synthetic hydrogelators
-
Gold Nanoparticles Decorated with Mannose-6-phosphate Analogues作者:Stéphanie Combemale、Jean-Norbert Assam-Evoung、Sabrina Houaidji、Rashda Bibi、Véronique Barragan-MonteroDOI:10.3390/molecules19011120日期:——Herein, the preparation of neoglycoconjugates bearing mannose-6-phosphate analogues is described by: (a) synthesis of a cyclic sulfate precursor to access the carbohydrate head-group by nucleophilic displacement with an appropriate nucleophile; (b) introduction of spacers on the mannose-6-phosphate analogues via Huisgen’s cycloaddition, the Julia reaction, or the thiol-ene reaction under ultrasound
-
Synthesis of peptide homo‐ and heterodimers as potential mimics of platelet‐derived growth factor BB作者:Louise A. Stubbing、Harveen Kaur、Sheryl X. Feng、Miranda Aalderink、Michael Dragunow、Margaret A. BrimbleDOI:10.1002/pep2.24150日期:2020.7disease. The platelet‐derived growth factor receptor β (PDGFRβ)/platelet‐derived growth factor BB (PDGF‐BB) signalling pathway is key to the regulation of pericyte survival and proliferation. A series of peptide dimers mimicking the ligand PDGF‐BB were prepared in the hope of stimulating PDGFRβ internalisation and activation of this pathway. Copper‐catalysed azide‐alkyne cycloaddition of peptide monomers
-
[EN] BCR-ABL TYROSINE-KINASE LIGANDS CAPABLE OF DIMERIZING IN AN AQUEOUS SOLUTION, AND METHODS OF USING SAME<br/>[FR] LIGANDS DE TYROSINE-KINASE BCR-ABL CAPABLES DE SE DIMÉRISER DANS UNE SOLUTION AQUEUSE, ET PROCÉDÉS D'UTILISATION DE CEUX-CI申请人:COFERON INC公开号:WO2015106292A1公开(公告)日:2015-07-16Described herein are monomers capable of forming a biologically useful multimer when in contact with one, two, three or more other monomers in an aqueous media. In one aspect, such monomers may be capable of binding to another monomer in an aqueous media (e.g. invivo) to form a multimer (e.g. a dimer). Contemplated monomers may include a ligand moiety, a linker element, and a connector element that joins the ligand moiety and the linker element. In an aqueous media, such contemplated monomers may join together via each linker element and may thus be capable of modulating one or more biomolecules substantially simultaneously, e.g., modulate two or more binding sites on a Bcr-Abl tyrosine kinase.
-
Mono- and dicationic short PEG and methylene dioxyalkylglycerols for use in synthetic gene delivery systems作者:Christopher A. Hurley、John B. Wong、Jimmy Ho、Michele Writer、Scott A. Irvine、M. Jayne Lawrence、Stephen L. Hart、Alethea B. Tabor、Helen C. HailesDOI:10.1039/b719702k日期:——A range of monocationic and dicationic dioxyalkylglycerol cytofectins have been synthesised possessing methylene and short n-ethylene glycol spacers. The monocationic compounds were found to be effective in transfections when formulated as lipopolyplexes with peptide and DNA components, in particular with shorter PEG head groups which may have less effect on peptide targeting in the ternary complex.
表征谱图
-
氢谱1HNMR
-
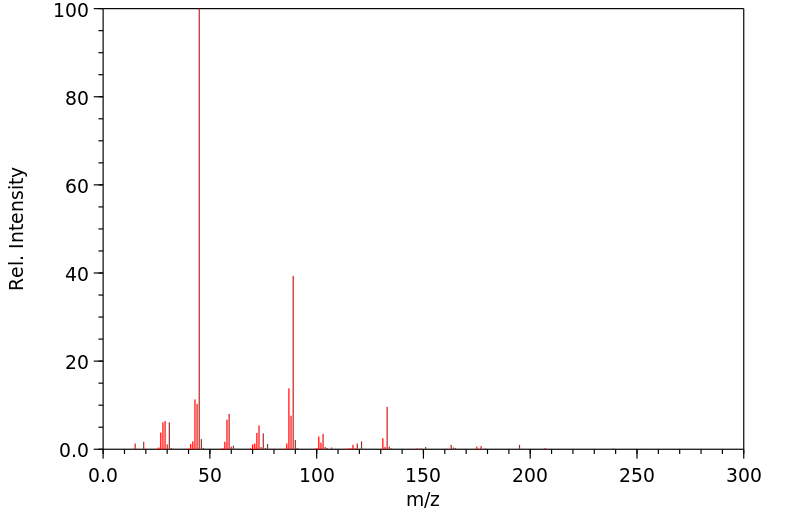
质谱MS
-
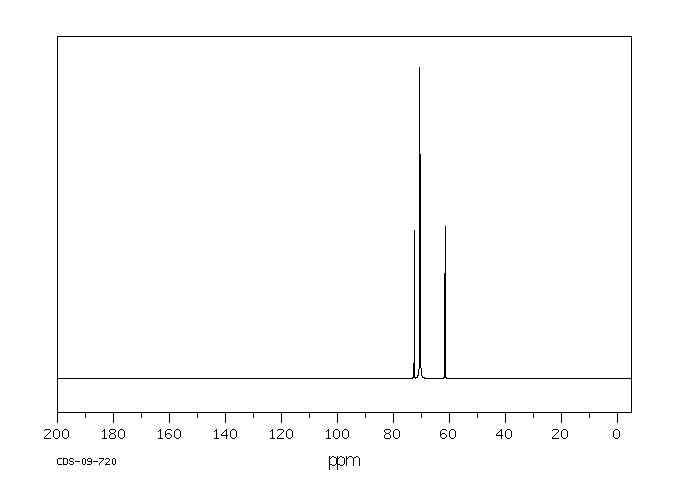
碳谱13CNMR
-
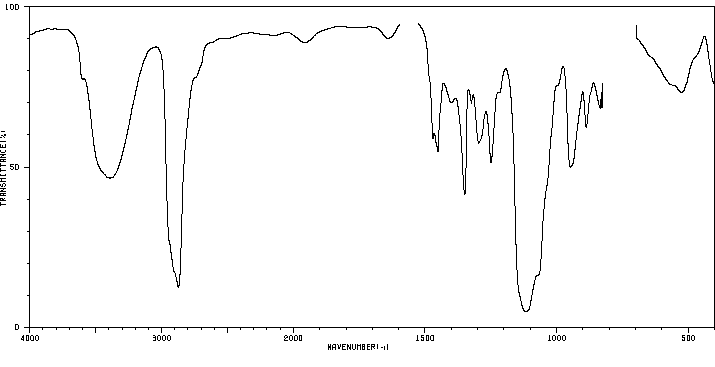
红外IR
-
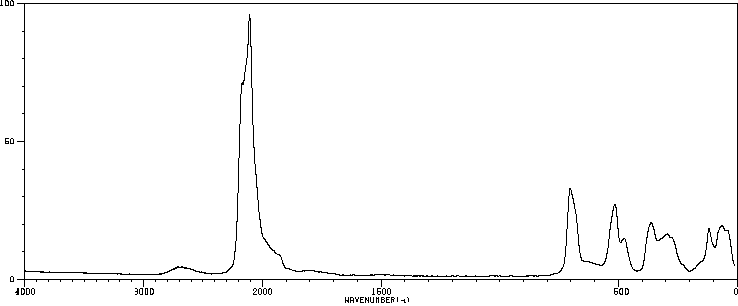
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷