硝基氯苯 | 88-73-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:241.15°C (rough estimate)
-
密度:1.3768 (rough estimate)
-
物理描述:O-nitrochlorobenzene appears as yellow crystals with an aromatic odor. Sinks in water. (USCG, 1999)
-
颜色/状态:Yellow crystals
-
熔点:32.5 °C
-
闪点:127 °C
-
溶解度:In water, 441 mg/L at 25 °C
-
蒸汽密度:5.44 (NTP, 1992) (Relative to Air)
-
蒸汽压力:0.018 mm Hg at 25 °C
-
亨利常数:9.30e-06 atm-m3/mole
-
自燃温度:470 °C
-
粘度:2.09X10-3 Pa-s at 317.65 deg K
-
汽化热:6.59X10+7 J/kmol at 306.14 deg K
-
表面张力:4.37X10-2 N/m at 317.65 deg K
-
保留指数:1193;1195;1221.4;1199
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:45.8
-
氢给体数:0
-
氢受体数:2
ADMET
安全信息
-
危险等级:6.1
-
安全说明:S28A,S36/37/39,S38,S45,S60
-
危险品运输编号:UN 1578 6.1/PG 2
-
WGK Germany:2
-
海关编码:29049085
-
危险类别:6.1
-
危险品标志:T
-
危险类别码:R22,R24,R52/53
-
RTECS号:CZ0875000
-
包装等级:II
SDS
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-氯-4-硝基苯胺 3-chloro-4-nitroaniline 825-41-2 C6H5ClN2O2 172.571 2,5-二氯硝基苯 2,5-dichloronitrobenzene 89-61-2 C6H3Cl2NO2 192.001 对硝基氯苯 4-chlorobenzonitrile 100-00-5 C6H4ClNO2 157.556 硝基苯 nitrobenzene 98-95-3 C6H5NO2 123.111 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,4-二氯硝基苯 2,4-dichloronitrobenzene 611-06-3 C6H3Cl2NO2 192.001 3-氯-4-硝基苯胺 3-chloro-4-nitroaniline 825-41-2 C6H5ClN2O2 172.571 2,5-二氯硝基苯 2,5-dichloronitrobenzene 89-61-2 C6H3Cl2NO2 192.001 2,3-二氯硝基苯 1,2-Dichloro-3-nitrobenzene 3209-22-1 C6H3Cl2NO2 192.001 3-氯-4-硝基苯酚 3-chloro-4-nitrophenol 491-11-2 C6H4ClNO3 173.556 2,6-二硝基氯苯 1-chloro-2,6-dinitrobenzene 606-21-3 C6H3ClN2O4 202.554 1-氯-2,4-二硝基苯 1-chloro-2,4-dinitro-benzene 97-00-7 C6H3ClN2O4 202.554 2-氯-5-碘硝基苯 1-chloro-4-iodo-2-nitrobenzene 41252-95-3 C6H3ClINO2 283.453 1-氯-2-亚硝基-苯 o-Chloronitrosobenzene 932-33-2 C6H4ClNO 141.557 5-溴-2-氯硝基苯 4-bromo-1-chloro-2-nitrobenzene 16588-24-2 C6H3BrClNO2 236.452 2-氯苯基羟胺 2-chloro-N-hydroxybenzenamine 10468-16-3 C6H6ClNO 143.573 2,4,5-三氯硝基苯 2,4,5-Trichloronitrobenzene 89-69-0 C6H2Cl3NO2 226.446 3-氯-4-硝基苯甲醛 3-chloro-4-nitrobenzaldehyde 57507-34-3 C7H4ClNO3 185.567 3-氯-2-硝基苯酚 3-chloro-2-nitrophenol 17802-02-7 C6H4ClNO3 173.556 1-氯-4-(氯甲基)-2-硝基苯 1-chloro-4-(chloromethyl)-2-nitrobenzene 57403-35-7 C7H5Cl2NO2 206.028 (3-氯-4-硝基苯基)乙腈 (3-Chloro-4-nitrophenyl)acetonitrile 80199-01-5 C8H5ClN2O2 196.593 硝基苯 nitrobenzene 98-95-3 C6H5NO2 123.111 1-氯-4-乙氧基-2-硝基苯 1-chloro-4-ethoxy-2-nitrobenzene 89979-06-6 C8H8ClNO3 201.609 1-氯-4-(4-氯-3-硝基苯基)-2-硝基苯 4,4'-dichloro-3,3'-dinitro-biphenyl 14470-81-6 C12H6Cl2N2O4 313.097 —— 2-(3-Chloro-4-nitrophenyl)propionitrile 86981-07-9 C9H7ClN2O2 210.62 —— 4,4'-Dichloro-3,3'-dinitrodiphenylmethane 2973-18-4 C13H8Cl2N2O4 327.124 —— 4-(3-chloro-4-nitrobenzyl)pyridine —— C12H9ClN2O2 248.669 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:Rikliss, 1940, vol. 7, p. 154摘要:DOI:
-
作为产物:参考文献:名称:芳基重氮盐的两相电化学生成:在电生成的铜催化的桑德迈尔反应中的应用。摘要:首次报道了在两相系统(乙酸乙酯/水)中由硝基芳烃电化学生成芳基重氮盐。以良好的收率和良好的纯度制备了包括偶氮,偶氮砜和芳基叠氮化物在内的一些化合物。阴极产生的芳基重氮和阳极产生的铜(I)离子用于进行Sandmeyer反应。为了改进该方法,引入了以Zn棒为阳极的H型自驱动电池,并将其用于两相芳基重氮的生产。DOI:10.1021/acs.orglett.0c02013
-
作为试剂:描述:参考文献:名称:元素硫通过氧化还原策略介导的环化:由邻氯硝基苯和苄基氯合成苯并噻唑摘要:已经开发了一种新的无金属的合成方法,该方法使用元素硫作为无痕氧化剂,由易于获得的邻氯硝基苯和苄基氯合成2-取代的苯并噻唑。该协议提供了一种简单,有效且原子经济的方法来以中等至优异的产率获得苯并噻唑。并且该方法表现出良好的官能团耐受性。DOI:10.1016/j.tet.2017.07.013
文献信息
-
Npy antagonists, preparation and uses申请人:Botez Iuliana公开号:US20090233910A1公开(公告)日:2009-09-17The present invention concerns novel compounds, their preparation and their uses, therapeutic uses in particular. More specifically it concerns derivative compounds having at least two aromatic cycles, their preparation and their uses, in particular in the area of human or animal health. These compounds have an affinity for the biological receptors of neuropeptide Y, NPY, present in the central and peripheral nervous systems. The compounds of the invention are preferably NPY antagonists, and more particularly antagonists of sub-type NPY Y1, and can therefore be used for the therapeutic or prophylactic treatment of any disorder involving NPY. The present invention also concerns pharmaceutical compositions containing said compounds, their preparation and their uses, as well as treatment methods using said compounds.本发明涉及新颖化合物,它们的制备和用途,特别是在治疗方面的用途。更具体地说,它涉及至少具有两个芳香环的衍生化合物,它们的制备和用途,特别是在人类或动物健康领域。这些化合物对存在于中枢和外周神经系统中的神经肽Y(NPY)的生物受体具有亲和力。本发明的化合物优选为NPY拮抗剂,更具体地说是NPY Y1亚型的拮抗剂,因此可用于治疗或预防涉及NPY的任何疾病。本发明还涉及含有所述化合物的药物组合物,其制备和用途,以及使用所述化合物的治疗方法。
-
Synthesis of near-infrared fluorescent rhodamines <i>via</i> an S<sub>N</sub>Ar<sup>H</sup> reaction and their biological applications作者:Qing Wang、Kun Huang、Songtao Cai、Chang Liu、Xiaojie Jiao、Song He、Liancheng Zhao、Xianshun ZengDOI:10.1039/c8ob01701h日期:——the preparation of a wide variety of π-extended near-infrared fluorescent rhodamine dyes. Using this strategy, seven rectilinearly π-extended rhodamines (RE1–RE7) that had fluorescence emission wavelengths in the near-infrared region were synthesized. RE1, RE3, and RE4 were lysosome targetable and showed good photostabilities. In addition, using dye RE1 as a precursor, we constructed a novel NIR fluorescent由于减少了来自生物样品的干扰吸收和荧光,减少了散射并提高了组织穿透深度,近红外(NIR)染料在生物医学中引起了人们的极大兴趣。在这种情况下,我们报告了使用芳香族氢的独特分子内亲核取代(S N Ar H)策略合成直线π延伸的若丹明染料的方法。该策略利用了预先组织的芳族氨基氮与x吨离子中电子不足的碳之间的S N Ar H反应。S N Ar H本文提出的反应可在没有过渡金属催化剂的温和条件下进行,并且有望实现多种π扩展的近红外荧光若丹明染料的制备。使用这种策略,合成了在近红外区域具有荧光发射波长的七个直线π扩展的若丹明(RE1-RE7)。RE1,RE3和RE4可溶酶体靶向,并显示出良好的光稳定性。此外,我们以染料RE1为前体,构建了一种新型的NIR荧光开启探针(RE1-Cu),可用于检测Cu 2+ 在活细胞中的含量,证明了我们近红外功能荧光染料的价值。
-
Efficient one-pot transformation of aminoarenes to haloarenes using halodimethylisulfonium halides generated in situ作者:Woonphil Baik、Wanqiang Luan、Hyun Joo Lee、Cheol Hun Yoon、Sangho Koo、Byeong Hyo KimDOI:10.1139/v05-026日期:2005.3.1
Halodimethylsulfonium halide 1, which is readily formed in situ from hydrohaloic acid and DMSO, is a good nucleophilic halide. This activated nucleophilic halide rapidly converts aryldiazonium salt prepared in situ by the same hydrohaloic acid and nitrite ion to aryl chlorides, bromides, or iodides in good yield. The combined action of nitrite ion and hydrohaloic acid in DMSO is required for the direct transformation of aromatic amines, which results in the production of aryl halides within 1 h. Substituted compounds with electron-donating or -withdrawing groups or sterically hindered aromatic amines are also smoothly transformed to the corresponding aromatic halides. The only observed by-product is the deaminated arene (usually <7%). The isolated aryldiazonium salts can also be converted to the corresponding aryl halides using 1. The present method offers a facile, one-step procedure for transforming aminoarenes to haloarenes and lacks the environmental pollutants that usually accompany the Sandmeyer reaction using copper halides. Key words: aminoarenes, haloarenes, halodimethylsulfonium halide, halogenation, amination.
卤二甲基亚砜卤化物1是一种良好的亲核卤化物,可在现场由氢卤酸和二甲亚砜形成。这种活化的亲核卤化物迅速将由相同的氢卤酸和亚硝酸根在现场制备的芳基重氮盐转化为芳基氯化物、溴化物或碘化物,收率较高。在DMSO中,亚硝酸根和氢卤酸的联合作用是直接转化芳香胺的必要条件,从而在1小时内产生芳基卤化物。带有电子给体或吸引基团或有立体位阻的芳香胺的取代化合物也可顺利转化为相应的芳香卤化物。观察到的唯一副产物是去氨基芳烃(通常<7%)。孤立的芳基重氮盐也可以使用1转化为相应的芳基卤化物。该方法提供了一种简便的、一步法的程序,用于将氨基芳烃转化为卤代芳烃,并且不伴随通常伴随使用铜卤化物进行桑迈尔反应的环境污染物。关键词:氨基芳烃,卤代芳烃,卤二甲基亚砜卤化物,卤化,胺化。 -
Highly chemoselective reduction of nitroarenes over non-noble metal nickel-molybdenum oxide catalysts作者:Haigen Huang、Xueguang Wang、Xu Li、Chenju Chen、Xiujing Zou、Weizhong Ding、Xionggang LuDOI:10.1039/c6gc03141b日期:——Chemoselective reduction of nitroarenes is an important transformation for the production of arylamines, which are the primary intermediates in the synthesis of pharmaceuticals, agrochemicals and dyes. Heterogeneous non-noble metal nickel-molybdenum...
-
Recyclable and Selective Nitroarene Hydrogenation Catalysts Based on Carbon-Coated Cobalt Oxide Nanoparticles作者:Bingfeng Chen、Fengbo Li、Zhijun Huang、Guoqing YuanDOI:10.1002/cctc.201501265日期:2016.3.18through direct heating treatment of cobalt oxide precursors incipiently deposited over nanographite materials. Cobalt oxides are partially reduced to active zero‐valent metal species and the simultaneous formation of carbon layers over the nanoparticles protects them from oxidation and deactivation. This nanocatalyst performs excellently in chemoselective hydrogenation of some challenging nitroarenes with
表征谱图
-
氢谱1HNMR
-
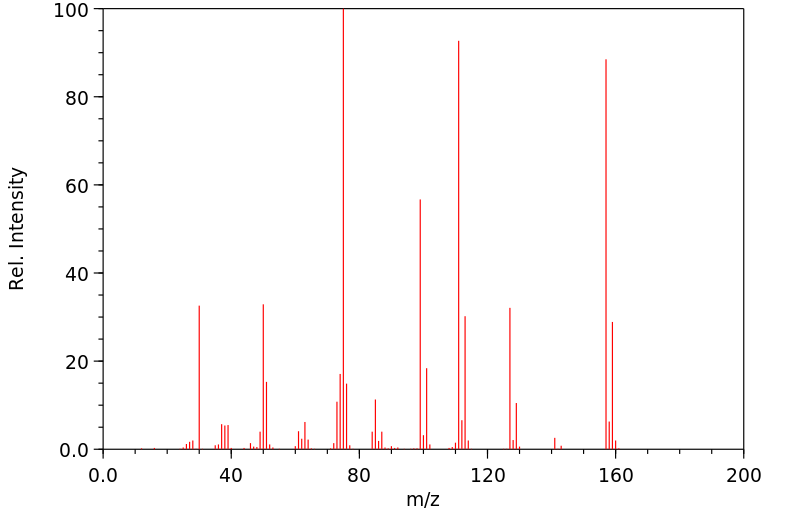
质谱MS
-
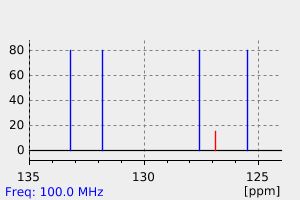
碳谱13CNMR
-
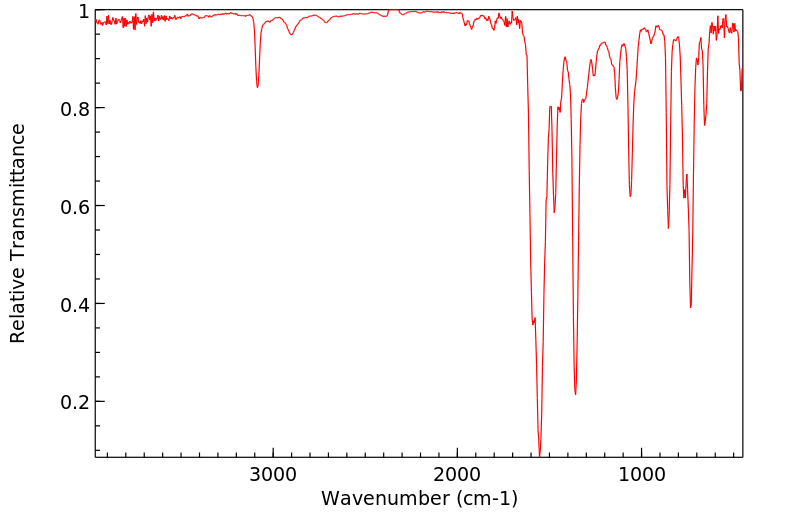
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息