2-乙酰氨基-2-脱氧-Alpha-D-吡喃糖 | 10036-64-3
中文名称
2-乙酰氨基-2-脱氧-Alpha-D-吡喃糖
中文别名
2-乙酰氨基-2-脱氧-ALPHA-D-吡喃葡萄糖;2-乙酰氨基-2-脱氧-alpha-d-吡喃葡萄糖;乙酰氨基-联氧-古氨酶;2-乙酰氨基-2-脱氧-α-D-吡喃糖
英文名称
N-acetyl-D-glucosamine
英文别名
N-acetylglucosamine;2-acetamido-2-deoxy-α-D-glucopyranose;GlcNAc;2-Acetamido-2-deoxy-alpha-D-glucopyranose;N-[(2S,3R,4R,5S,6R)-2,4,5-trihydroxy-6-(hydroxymethyl)oxan-3-yl]acetamide
CAS
10036-64-3
化学式
C8H15NO6
mdl
——
分子量
221.21
InChiKey
OVRNDRQMDRJTHS-PVFLNQBWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:211 °C
-
沸点:595.4±50.0 °C(Predicted)
-
密度:1.50
-
LogP:-2.314 (est)
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):-1.7
-
重原子数:15
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.88
-
拓扑面积:119
-
氢给体数:5
-
氢受体数:6
安全信息
-
危险品标志:Xi
-
安全说明:S24/25
-
海关编码:29329900
-
储存条件:存放在4°C阴凉干燥处
SDS
| Name: | 2-Acetamido-2-Deoxy-alpha-D-Glucopyranose 99.5+% Material Safety Data Sheet |
| Synonym: | N-Acetyl-alpha-D-glucosamine |
| CAS: | 10036-64-3 |
Synonym:N-Acetyl-alpha-D-glucosamine
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 10036-64-3 | 2-Acetamido-2-Deoxy-alpha-D-Glucopyran | 99.5 | 233-115-1 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
Ingestion of large amounts may cause gastrointestinal irritation.
The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Keep container closed when not in use.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate general or local exhaust ventilation to keep airborne concentrations below the permissible exposure limits.
Exposure Limits CAS# 10036-64-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves and clothing to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white to off-white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 211 deg C
Autoignition Temperature: Not available.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: 211 deg C
Solubility in water: soluble
Specific Gravity/Density:
Molecular Formula: C8H15NO6
Molecular Weight: 221.21
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Dust generation.
Incompatibilities with Other Materials:
Oxidizing agents, strong acids, strong bases.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 10036-64-3 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-Acetamido-2-Deoxy-alpha-D-Glucopyranose - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing group:
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 10036-64-3: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 10036-64-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 10036-64-3 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-乙酰氨基-2-脱氧-beta-D-吡喃葡萄糖胺 D-N-acetylglucosamine 14131-68-1 C8H15NO6 221.21 —— N,N',N'',N'''-tetraacetylchitotetraose —— C32H54N4O21 830.795 —— O-(2-acetamido-2-deoxy-β-D-glucopyranosyl)-(1->4)-O-(2-acetamido-2-deoxy-β-D-glucopyranosyl)-(1->4)-2-acetamido-2-deoxy-D-glucopyranose 13319-32-9 C24H41N3O16 627.6 —— 2-deoxy-2-amino glucose 3416-24-8 C6H13NO5 179.173 —— 4,6-dimethoxy-1,3,5-triazin-2-yl α-N-acetylglucosaminide 1337923-38-2 C13H20N4O8 360.324 —— 19-(2-acetamido-2-deoxy-α-D-glucopyranosyloxy)isopimara-7,15-dien-3β-ol 1320356-11-3 C28H45NO7 507.668 —— p-nitrophenyl 2-acetamido-4-O-(2-acetamido-2-deoxy-β-D-glucopyranosyl)-2-deoxy-β-D-glucopyranoside 7284-16-4 C22H31N3O13 545.5 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— N-acetyl-beta-D-galactosamine 14131-60-3 C8H15NO6 221.21 2-乙酰胺基-2-脱氧-D-半乳糖 N-acetyl-D-galactosamine 14215-68-0 C8H15NO6 221.21 2-乙酰氨基-2-脱氧-beta-D-吡喃葡萄糖胺 D-N-acetylglucosamine 14131-68-1 C8H15NO6 221.21 —— methyl N-acetyl-D-glucosamine —— C9H17NO6 235.237 甲基 2-乙酰氨基-2-脱氧-alpha-D-吡喃葡萄糖苷 methyl 2-acetylamido-2-deoxy-D-glucoside 6082-04-8 C9H17NO6 235.237 —— methyl 2-acetamido-2-deoxy-α-D-allopyranoside 114612-10-1 C9H17NO6 235.237 —— isopropyl 2-acetamido-2-deoxy-α-D-glucopyranoside 19124-40-4 C11H21NO6 263.291 烯丙基 2-乙酰氨基-2-脱氧-alpha-D-吡喃葡萄糖苷 allyl 2-acetamido-2-deoxy-α-D-glucopyranoside 54400-75-8 C11H19NO6 261.275 —— allyl 2-acetamido-2-deoxy-β-D-glucopyranoside —— C11H19NO6 261.275 —— 2-chloroethyl 2-acetamido-2-deoxy-α-D-glucopyranoside 2495-94-5 C10H18ClNO6 283.709 —— 2-azidoethyl 2-acetamido-2-deoxy-α-D-glucopyranoside 593252-73-4 C10H18N4O6 290.276 —— 2-azidoethyl 2-N-acetyl-2-deoxy-α-D-galactopyranoside 195384-42-0 C10H18N4O6 290.276 —— allyl 2-acetamido-2-deoxy-4,6-O-isopropylidene-α-D-glucopyranoside 95069-09-3 C14H23NO6 301.34 苄基-2-乙酰胺基-2-脱氧-Alpha-D-吡喃葡萄糖苷 benzyl 2-acetamido-2-deoxy-α-D-glucopyranoside 13343-62-9 C15H21NO6 311.335 —— benzyl 2-deoxy-2-(N-acetylamino)-D-glucopyranoside —— C15H21NO6 311.335 —— N-acetylglucosamine-1-phosphate 28446-21-1 C8H16NO9P 301.19 β-D-葡萄糖胺五乙酸酯 2-acetamido-1,3,4,6-tetra-O-acetyl-2-deoxy-β-D-glucopyranose 7772-79-4 C16H23NO10 389.359 A-D-氨基葡萄糖五乙酸盐 Acetic acid (2R,3R,4R,5S,6R)-2,5-diacetoxy-6-acetoxymethyl-3-acetylamino-tetrahydro-pyran-4-yl ester 7784-54-5 C16H23NO10 389.359 —— 2-azidoethyl 2-acetamido-3,4,6-tri-O-acetyl-2-deoxy-β-D-glucopyranoside 142072-11-5 C16H24N4O9 416.388 —— 2-acetamido-1,3,4,6-tetra-O-acetyl-2-deoxy-α-D-galactopyranoside 10385-50-9 C16H23NO10 389.359 2-脱氧-2-氨基-A-D-葡萄糖 glucosamine 6490-70-6 C6H13NO5 179.173 —— 4,6-dimethoxy-1,3,5-triazin-2-yl α-N-acetylglucosaminide 1337923-38-2 C13H20N4O8 360.324 苄基(5Xi)-2-乙酰氨基-2-脱氧-4,6-O-异亚丙基-alpha-D-来苏-吡喃己糖苷 N-((4aR,6S,7R,8R,8aS)-6-(benzyloxy)-8-hydroxy-2,2-dimethylhexahydropyrano[3,2-d][1,3]dioxin-7-yl)acetamide 66026-10-6 C18H25NO6 351.4 —— methyl 2-acetamido-4,6-O-benzylidene-2-deoxy-α-D-allopyranoside 6619-02-9 C16H21NO6 323.346 —— N-((4aR,6S,7R,8R,8aS)-8-Hydroxy-6-methoxy-2-phenyl-hexahydro-pyrano[3,2-d][1,3]dioxin-7-yl)-acetamide 6619-04-1 C16H21NO6 323.346 苄基 2-(乙酰氨基)-2-脱氧-6-O-(苯基甲基)-3-O-2-丙烯-1-基-alpha-D-吡喃葡萄糖苷 benzyl 2-acetamido-3-O-allyl-6-O-benzyl-2-deoxy-α-D-glucopyranoside 60920-82-3 C25H31NO6 441.524 —— ((4aR,6S,7R,8R,8aS)-6-Allyloxy-8-hydroxy-2,2-dimethyl-hexahydro-pyrano[3,2-d][1,3]dioxin-7-yl)-carbamic acid tert-butyl ester 293328-84-4 C17H29NO7 359.42 —— 6-O-p-methylbenzenesulfonate-N-acetylglucosamine 121363-58-4 C15H21NO8S 375.4 —— N-acetylneuraminic acid 140850-44-8 C11H19NO9 309.273 —— benzyl 2-acetamido-4,6-O-benzylidene-2-deoxy-α-D-glucopyranoside 78246-81-8 C22H25NO6 399.444 2-乙酰氨基-1-氨基-1,2-二脱氧-beta-D-吡喃葡萄糖 2-acetamido-2-deoxy-β-D-glucopyranosylamine 4229-38-3 C8H16N2O5 220.225 —— allyl 2-acetamido-4,6-O-(4-fluorobenzylidene)-2-deoxy-α-D-glucopyranoside 613672-67-6 C18H22FNO6 367.374 —— allyl 2-amino-2-deoxy-α-D-glucopyranoside 112146-92-6 C9H17NO5 219.238 —— 2-acetamido-2-deoxy-1,3,4,6-tetra-O-trimethylsilyl-α-D-glucopyranose 53110-68-2 C20H47NO6Si4 509.938 —— i-propyl 1-thio-4-O-(2-acetamido-2-deoxy-α-glucopyranosyl)-β-galactopyranoside 1352031-32-3 C17H31NO10S 441.5 —— Bn(-2)[Bn(-3)][Bn(-4)]Fuc(a1-3)[Bn(-6)Gal(b1-4)][Bn(-6)]GlcNAc(a)-O-Bn 136543-98-1 C62H71NO15 1070.24 —— 2-chloroethyl 2-acetamido-3,6-di-O-benzoyl-2-deoxy-α-D-glucopyranoside 593252-74-5 C24H26ClNO8 491.925 —— 2-azidoethyl 2-acetamido-3,6-di-O-benzoyl-2-deoxy-α-D-glucopyranoside 593252-75-6 C24H26N4O8 498.492 —— allyl 2-amino-2-deoxy-4,6-O-isopropylidene-α-D-glucopyranoside 138527-55-6 C12H21NO5 259.302 —— 2-azidoethyl 2-acetamido-3,4,6-tri-O-benzoyl-2-deoxy-α-D-galactopyranoside 593252-76-7 C31H30N4O9 602.601 —— Benzyl 2-acetamido-2-deoxy-4,6-O-isopropylidene-3-O-[(methylsulfanyl)thiocarbonyl]-α-D-glucopyranoside 192065-17-1 C20H27NO6S2 441.569 —— 2-acetamido-1,3,4-tri-O-acetyl-2-deoxy-6-O-(triphenylmethyl)-α-D-glucopyranose 10026-54-7 C33H35NO9 589.642 2-(乙酰氨基)-2-脱氧-D-山梨糖醇 2-acetylamino-2-deoxy-D-glucitol 4271-28-7 C8H17NO6 223.226 —— 2-acetamido-2-deoxy-3-O-carbamoyl-α-D-glucopyranos-1-yl (2'R')-2'-carboxy-2'-(pentyloxy)-eth-1'-yl phosphate 128753-52-6 C17H31N2O13P 502.412 —— methyl 2-acetamido-4,6-O-benzylidene-3-O-mesyl-2-deoxy-α-D-glucopyranoside 6619-03-0 C17H23NO8S 401.438 —— benzyl 2-acetamido-3-C-allyl-2,3-dideoxy-4,6-O-isopropylidene-α-D-glucopyranoside 716377-08-1 C21H29NO5 375.465 —— allyl 2-acetamido-3-O-(2-fluorobenzoyl)-4,6-O-(4-fluorobenzylidene)-2-deoxy-α-D-glucopyranoside 613672-68-7 C25H25F2NO7 489.473 2-乙酰氨基-3,4,6-三-O-乙酰-2-脱氧-α-D-吡喃葡萄糖酰基氯 Acetic acid (2R,3S,4R,5R,6R)-3-acetoxy-2-acetoxymethyl-5-acetylamino-6-chloro-tetrahydro-pyran-4-yl ester 3068-34-6 C14H20ClNO8 365.768 - 1
- 2
- 3
- 4
- 5
反应信息
-
作为反应物:描述:2-乙酰氨基-2-脱氧-Alpha-D-吡喃糖 在 sodium hydride 、 对甲苯磺酸 、 N,N-二甲基甲酰胺 、 原甲酸三乙酯 作用下, 以 1,4-二氧六环 、 苯 为溶剂, 反应 34.0h, 生成 benzyl 2-acetamido-4,6-O-benzylidene-2-deoxy-3-O-[(R)-1-(methoxycarbonyl)ethyl]-α-D-glucopyranoside参考文献:名称:An improved total synthesis of UDP-N-acetyl-muramic acid摘要:Biochemical testing for novel inhibitors of Mur ligases requires several commercially unavailable and structurally complex substrates. We describe a modified synthetic strategy for the total chemical synthesis of the MurC ligase substrate UDP-Nacetyl-muramic acid which includes several improvements over published methods, especially with regard to purification procedures. The synthetic strategy is applicable for the synthesis of further Mur ligase substrates. (c) 2007 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2007.04.098
-
作为产物:描述:p-nitrophenyl 2-acetamido-4-O-(2-acetamido-2-deoxy-β-D-glucopyranosyl)-2-deoxy-β-D-glucopyranoside 在 salmonella chitinase 作用下, 反应 3.33h, 生成 2-乙酰氨基-2-脱氧-Alpha-D-吡喃糖参考文献:名称:新型鼠伤寒沙门氏菌几丁质酶的表征,该酶水解几丁质,壳寡糖和N-乙酰基乳糖胺共轭物摘要:沙门氏菌含有注释为几丁质酶的基因。然而,它们的几丁质分解活性从未得到证实。现在,我们证明了针对分配给鼠伤寒沙门氏菌SL1344中SL0018(chiA)基因编码的糖苷水解酶家族18的几丁质酶的这种活性。通过PCR扩增缺乏假定的几丁质结合域的chiA的C末端截短形式,克隆并在具有N末端(His)6标签的大肠杆菌BL21(DE3)中表达。纯化的酶水解4-硝基苯基N,N'-二乙酰基-β- d-壳聚糖,4-硝基苯基β- d - N,Ñ ',Ñ “-triacetylchitotriose和羧甲基甲壳素雷玛唑亮紫但不作用4-硝基苯基ñ -乙酰基β- d -glucosaminide,肽聚糖或4-硝基苯基β- d -cellobioside。酶活性的特征还在于使用1 H核磁共振直接监测产物的形成,这表明几丁质是释放N,N'-二乙酰基壳二糖的底物。水解在保留构型的情况下发生,并且该酶仅作用于壳寡糖底物的DOI:10.1093/glycob/cwq174
-
作为试剂:描述:4-methylumbelliferyl tetra-N-acetyl-β-chitotetraoside 在 2-乙酰氨基-2-脱氧-Alpha-D-吡喃糖 、 hen eggwhite (HEW) lysozyme 、 水 作用下, 反应 2.0h, 生成 羟甲香豆素参考文献:名称:Hydrolysis of 4-methylumbelliferyl tetra-N-acetyl-.BETA.-chitotetraoside by lysozyme and its inhibition by N,N',N"-triacetylchitotriose.摘要:溶菌酶对4-甲基伞形酮四-N-乙酰-β-壳四糖(4-MU-(GlcNAc)4)的水解活性受离子强度的影响很小,尽管溶菌酶对微球菌悬浮液的水解活性随离子强度的变化而有显著差异。0.1mM的N, N', N"-三乙酰壳三糖((GlcNAc)3)抑制了溶菌酶对4-MU-(GlcNAc)4作为底物的40-60%活性,但溶菌酶对微球菌的溶解活性影响不大。研究了鸡蛋清(HEW)溶菌酶和人胎盘(HP)溶菌酶对4-MU-(GlcNAc)4的水解动力学以及(GlcNAc)3对此水解的抑制作用。HEW溶菌酶和HP溶菌酶对4-MU-(GlcNAc)4的K5值分别为19.7μM和27.9μM,而Vmax值分别为0.124和0.0833 nmol/min/mg。两种酶的k值都很低,暗示底物分子中的切割键相对于溶菌酶活性位点的取向不佳。(GlcNAc)3以双曲线混合型抑制作用抑制溶菌酶。抑制作用降低了两种溶菌酶的Vmax值。加入抑制剂后,HEW溶菌酶的K5值增加,而HP溶菌酶的K5值降低。HEW溶菌酶和HP溶菌酶的Ki值分别为29.6μM和106μM。DOI:10.1248/cpb.32.3607
文献信息
-
PHOSPHORAMIDITE BUILDING BLOCKS FOR SUGAR-CONJUGATED OLIGONUCLEOTIDES申请人:AM CHEMICALS LLC公开号:US20160083414A1公开(公告)日:2016-03-24Novel nucleoside phosphoramidite building blocks for preparation of synthetic oligonucleotides containing at least one phosphotriester linkage conjugated to a monosaccharide and synthetic processes for making the same are disclosed. Furthermore, oligomeric compounds are prepared using said building blocks, preferably followed by removal of protecting groups to provide monosaccharide-conjugated oligonucleotides.
-
[EN] IMIDAZOLIUM REAGENT FOR MASS SPECTROMETRY<br/>[FR] RÉACTIF D'IMIDAZOLIUM POUR SPECTROMÉTRIE DE MASSE申请人:HOFFMANN LA ROCHE公开号:WO2021234004A1公开(公告)日:2021-11-25The present invention relates to compounds which are suitable to be used in mass spectrometry as well as methods of mass spectrometric determination of analyte molecules using said compounds.本发明涉及适用于质谱的化合物,以及利用该化合物进行分析物分子的质谱测定方法。
-
HUMAN iNKT CELL ACTIVATION USING GLYCOLIPIDS
-
INTRACELLULAR SUBSTANCE TRANSPORT SYSTEM AND USE THEREOF申请人:National University Corporation Hokkaido University公开号:US20200138973A1公开(公告)日:2020-05-07The present invention pertains to a complex including nanoparticles and, carried on the surface of the nanoparticles, a lysosomal enzyme inhibitor or kinase inhibitor shown by general formula (A) and a phospholipid mimetic substance shown by general formula (B). In general formula (A), n1 is an integer of 2-30, n2 is an integer of 2-30, the -S- terminal is a nanoparticle-carrying site, and R10 is a suicide substrate site or a kinase inhibition site. In general formula (B), n3 is an integer in the range of 2-30, and the -S- terminal is a nanoparticle-carrying site. The present invention provides a versatile system capable of efficiently delivering a drug to endolysosomes and allowing the drug to function at a low concentration on lysosomal enzymes, and an anticancer agent in which this system is used.
-
CHITOSAN COVALENTLY LINKED WITH SMALL MOLECULE INTEGRIN ANTAGONIST FOR TARGETED DELIVERY申请人:Hoffmann-La Roche Inc.公开号:US20130197205A1公开(公告)日:2013-08-01The invention relates to the chitosan polymer derivatives of formula I: and pharmaceutically acceptable salts and esters thereof, wherein Y, X 1 , X 4 , R1, R2, and n are defined in the detailed description and claims. The chitosan polymer derivatives of formula I bind to or associate with alpha-4-beta-1 (α4β1) and alpha-V-beta-3 (αVβ3) integrin dimers and can be used in delivery formulations to deliver drugs, nucleic acids, or other therapeutic compounds to tissues or cells expressing such integrins.
表征谱图
-
氢谱1HNMR
-
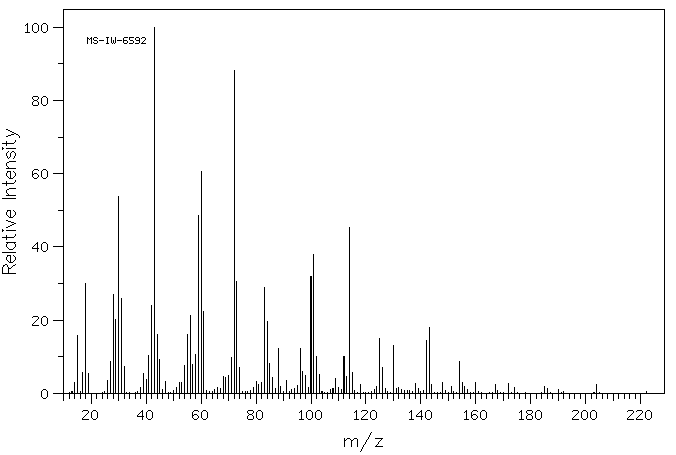
质谱MS
-
碳谱13CNMR
-
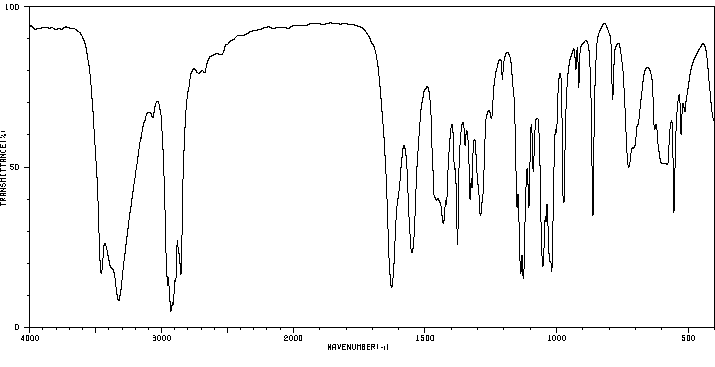
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷