3-甲基苯胺 | 108-44-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-30 °C
-
沸点:203-204 °C(lit.)
-
密度:0.999 g/mL at 25 °C(lit.)
-
闪点:186 °F
-
溶解度:0.2g/100ml(20°C)
-
介电常数:5.9500000000000002
-
暴露限值:TLV-TWA skin 2-ppm (~9 mg/m3) (ACGIH).
-
LogP:1.4 at 25℃
-
物理描述:M-toluidine appears as a clear colorless liquid. Flash point below 200°F. Vapors heavier than air. Toxic by inhalation, ingestion, and skin absorption in high concentrations or under prolonged exposures. Used in the manufacture of organic chemicals. Density about 8 lb / gal.
-
颜色/状态:Colorless to light yellow liquid
-
气味:Aromatic, amine-like odor
-
蒸汽密度:3.90 (Air = 1)
-
蒸汽压力:0.303 mm Hg at 25 °C
-
亨利常数:1.66e-06 atm-m3/mole
-
自燃温度:481 °C (898 °F)
-
分解:When heated to decomposition it emits highly toxic fumes of /nitroxides/.
-
粘度:3.306 mPa-sec at 25 °C
-
汽化热:44.9 kJ/mol at 203.3 °C
-
电离电位:7.50 eV
-
折光率:Index of refraction: 1.5681 at 20 °C/D
-
解离常数:pKa = 4.69 at 25 °C (conjugate acid)
-
保留指数:1047.5;1064;1064;1088;1088;1060;1060;1043
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:26
-
氢给体数:1
-
氢受体数:1
ADMET
安全信息
-
TSCA:Yes
-
危险等级:6.1
-
危险品标志:T
-
安全说明:S28,S28A,S36/37,S45,S53,S61
-
危险类别码:R23/24/25,R50,R33
-
WGK Germany:2
-
海关编码:29214300
-
危险品运输编号:UN 1708 6.1/PG 2
-
危险类别:6.1
-
RTECS号:XU2800000
-
包装等级:II
-
储存条件:储存注意事项: - 储存于阴凉、通风的库房。 - 远离火种、热源,包装要求密封,不可与空气接触。 - 应与氧化剂、酸类及食用化学品分开存放,切忌混储。 - 配备相应品种和数量的消防器材。 - 储区应备有泄漏应急处理设备和合适的收容材料。
SDS
| 国标编号: | 61750 |
| CAS: | 108-44-1 |
| 中文名称: | 3-甲基苯胺 |
| 英文名称: | 3-Toluidine;m-Toluidine |
| 别 名: | 间甲苯胺;3-氨基甲苯;间氨基甲苯 |
| 分子式: | C 7 H 9 N;CH 3 C 6 H 4 NH 2 |
| 分子量: | 107.15 |
| 熔 点: | -50.5℃ 沸点:203.3? |
| 密 度: | 相对密度(水=1)0.99; |
| 蒸汽压: | 85℃ |
| 溶解性: | 微溶于水,溶于醇、醚、稀酸 |
| 稳定性: | 稳定 |
| 外观与性状: | 无色油状液体 |
| 危险标记: | 14(毒害品) |
| 用 途: | 用作制造还原染料的中间体 |
2.对环境的影响:
一、健康危害
侵入途径:吸入、食入、经皮吸收。
健康危害:本品是强烈的高铁血红蛋白形成剂,并能刺激膀胱尿道,能致血尿。
急性中毒:多由皮肤污染而吸收。引起自觉脸部灼热、剧烈头痛、头晕、呼吸困难,呈现紫绀症。以后出现血尿、尿闭、精神障碍、肌肉抽搐。
慢性中毒时,可引起膀胱刺激症状。
二、毒理学资料及环境行为
毒性:属中等毒类。
急性毒性:LD50450mg/kg(大鼠经口);150mg/kg(小鼠腹腔)
致癌性:大鼠经口最小中毒剂量6600μg/kg(19周,间断)致肿瘤阳性。
危险特性:遇明火、高热或与氧化剂接触,有引起燃烧爆炸的危险。受高热分解放出有毒气体。
燃烧(分解)产物:一氧化碳、二氧化碳、氧化氮。
3.现场应急监测方法:
4.实验室监测方法:
对二甲氨基甲醛比色法《空气中有害物质的测定方法》(第二版)杭士平主编
对硝基重氮苯比色法《空气中有害物质的测定方法》(第二版)杭士平主编
气相色谱法《空气中有害物质的测定方法》(第二版)杭士平主编
5.环境标准:
前苏联 车间空气中有害物质的最高容许浓度 3mg/m3[皮]
6.应急处理处置方法:
一、泄漏应急处理
疏散泄漏污染区人员至安全区,禁止无关人员进入污染区,建议应急处理人员戴自验式呼吸器,穿厂商特别推荐的化学防护服(完全隔离)。不要直接接触泄漏物,在确保安全情况下堵漏。喷雾状水,减少蒸发。用沙土或其它不燃性吸附剂混合吸收,然后收集运至废物处理场所处置。也可以用大量水冲洗,经稀释的洗水放入废水系统。如大量泄漏,利用围堤收容,然后收集、转移、回收或无害处理后废弃。
二、防护措施
呼吸系统防护:可能接触其蒸气时,佩带防毒面具。紧急事态抢救或逃生时,佩带正压自给式呼吸器。
眼睛防护:戴安全防护眼镜。
防护服:穿紧袖工作服,长统胶鞋。
手防护:戴橡皮手套。
其它:工作现场禁止吸烟、进食和饮水。及时换洗工作服。工作前后不饮酒,用温水洗澡。监测毒物。进行就业前和定期的体检。
三、急救措施
皮肤接触:立即脱去污染的衣着,用肥皂水及清水彻底冲洗。注意手、足和指甲等部位。
眼睛接触:立即提起眼睑,用大量流动清水或生理盐水冲洗。
吸入:迅速脱离现场至空气新鲜处。呼吸困难时给输氧。呼吸停止时,立即时进行人工呼吸。就医。
食入:误服者给漱口,饮水,洗胃后口服活性炭,再给以导泻。就医。
灭火方法:雾状水、泡沫、二氧化碳、干粉、砂土。
制备方法与用途
3-甲基苯胺(间甲苯胺)是一种无色液体,在空气中或光照下会变为黄色或红棕色。其相对密度为0.989,熔点为-43.6℃,沸点在203~204℃之间。微溶于水,能溶于乙醇和乙醚,并能随水蒸气一同挥发。它由间硝基甲苯经还原反应制得,主要用作医药合成中间体。
应用甲基苯胺是应用广泛的有机合成中间体之一,主要用于染料、农药及医药等领域。目前在国内生产中,主要是以硝基甲苯为原料,乙醇或甲醇作为溶剂,并采用骨架Ni催化剂进行加氢反应制备甲基苯胺。
制备将间硝基甲苯和纳米催化剂加入高压反应釜中,并用氮气置换3次(压力0.3MPa),启动搅拌。开启氢气钢瓶减压阀和调压阀,控制氢气压力并充入适量氢气后加热升温至设定温度,持续搅拌通入氢气同时向反应釜内通冷却水移走反应热,维持稳定反应温度直到反应釜压力开始上升;停止通氢和冷却水继续搅拌加热至0.5小时;停止搅拌分离催化剂及反应液,在合适的条件下提取30%(质量)的反应液,称重并分析主含量。采用甲基苯胺作为溶剂与纳米催化剂催化加氢制备间甲苯胺工艺可行,且催化剂和溶剂可循环使用。此过程安全环保成本低能耗小;最佳工艺指标为:硝基甲苯与甲基苯胺质量比4∶10、纳米催化剂加入量为硝基甲苯的10%、反应温度100~120℃、氢气压力1.20~1.40MPa、搅拌速率1500r/min。在此条件下,硝基甲苯转化率可达88.5%以上,选择性达100%。
合成方法利用镍为催化剂,在工业甲醇溶剂中催化间硝基甲苯加氢反应生成3-甲基苯胺。通过单因素考查与正交实验确定最佳工艺条件:以80g间硝基甲苯为基础量,固定在200mL的工业甲醇溶剂条件下,催化剂用量为2.0g、反应时间为6小时、反应温度90℃、氢气压力1.6MPa。此时,3-甲基苯胺含量为99.79%,轻组分含量仅为0.11%而重组分则占0.10%。
化学性质3-甲基苯胺是一种无色油状液体,熔点为-43.6℃,沸点203.2℃,相对密度0.990(25/25℃),折光率1.8686,闪点为85℃。它能溶于醇、醚和稀酸但微溶于水,并随水蒸气挥发。在空气或光照下会变色。
用途3-甲基苯胺用作分析试剂,也用于有机合成及染料制造。作为活性黄X-R、阳离子紫2RL的中间体可用来检定锇、钯、铂、钌和亚硝酸盐等元素。
生产方法 类别有毒物品
毒性分级高毒
急性毒性口服:大鼠 LD50: 450 毫克/公斤;小鼠 LD50: 740 毫克/公斤
刺激数据皮肤接触:兔子,500毫克/24小时 轻度刺激; 眼睛接触:兔子,20毫克/24小时 中度刺激
可燃性危险特性遇明火可燃烧;生成有毒氮氧化物烟雾;与氧化剂反应
储运特性库房应保持通风、低温及干燥状态;不得与氧化剂、酸类或食品添加剂存放于同一仓库内
灭火方法使用二氧化碳、泡沫灭火器、砂土或干粉扑灭
职业卫生标准时间加权平均容许浓度为9毫克/立方米(TWA)
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-氨基-3,5-二甲苯 3,5-dimethylaminoaniline 108-69-0 C8H11N 121.182 乙烷,三氯氟- p-toluidine 106-49-0 C7H9N 107.155 3-甲苯肼 m-tolylhydrazine 536-89-0 C7H10N2 122.17 间甲苯基羟胺 N-(3-methylphenyl)hydroxylamine 620-25-7 C7H9NO 123.155 3-亚硝基甲苯 3-nitrosotoluene 620-26-8 C7H7NO 121.139 3-(甲氨基)甲苯 N-methyl-m-toluidine 696-44-6 C8H11N 121.182 —— 1-azido-3-methylbenzene 4113-72-8 C7H7N3 133.153 3-甲基N-甲酰苯胺 N-formyl-m-toluidine 3085-53-8 C8H9NO 135.166 —— N'-(3-methylphenyl)methanimidamide 94793-44-9 C8H10N2 134.181 4-氯-3-甲基苯胺 3-methyl-4-chloroaniline 7149-75-9 C7H8ClN 141.6 —— 3,3'-dimethylhydrazobenzene 621-26-1 C14H16N2 212.294 3,3'-偶氮甲苯 3,3'-dimethylazobenzene 588-04-5 C14H14N2 210.279 —— phenyl-m-tolyl-diazene 17478-66-9 C13H12N2 196.252 —— trans-3,3'-dimethylazobenzene 51437-67-3 C14H14N2 210.279 N-异丙基间甲苯胺 N-isopropyl-3-methylaniline 10219-26-8 C10H15N 149.236 2-氨基-4-甲基苯甲腈 5-methyl-2-cyanoaniline 26830-96-6 C8H8N2 132.165 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3,4-二甲基苯胺 4-amino-o-xylene 95-64-7 C8H11N 121.182 3,4-二氨基甲苯 4-methyl-1,2-diaminobenzene 496-72-0 C7H10N2 122.17 2,5-二甲基苯胺 2,5-Dimethylaniline 95-78-3 C8H11N 121.182 2,5-二氨基甲苯 2-methyl-p-phenylenediamine 95-70-5 C7H10N2 122.17 乙烷,三氯氟- p-toluidine 106-49-0 C7H9N 107.155 3-甲苯肼 m-tolylhydrazine 536-89-0 C7H10N2 122.17 3-氨基苯甲醛 m-aminobenzaldehyde 1709-44-0 C7H7NO 121.139 3-亚硝基甲苯 3-nitrosotoluene 620-26-8 C7H7NO 121.139 间甲苯基羟胺 N-(3-methylphenyl)hydroxylamine 620-25-7 C7H9NO 123.155 3-(甲氨基)甲苯 N-methyl-m-toluidine 696-44-6 C8H11N 121.182 邻甲苯胺 o-toluidine 95-53-4 C7H9N 107.155 2,3-二甲基苯胺 2,3-Dimethylaniline 87-59-2 C8H11N 121.182 —— N,N-dichloro-m-toluidine 861559-76-4 C7H7Cl2N 176.045 异氰酸间甲苯酯 3-tolyl isocyanate 621-29-4 C8H7NO 133.15 —— N-Sulfinyl-m-toluidin 15795-43-4 C7H7NOS 153.205 N-乙基间甲苯胺 N-ethyl-m-toulidine 102-27-2 C9H13N 135.209 间甲苯异硫氰酸酯 3-methylphenyl isothiocyanate 621-30-7 C8H7NS 149.216 —— N,N-dideuterio-3-methyl-aniline 39274-91-4 C7H9N 109.139 3-甲基N-甲酰苯胺 N-formyl-m-toluidine 3085-53-8 C8H9NO 135.166 N,N-二甲基-间甲基苯胺 N,N-dimethyl-m-toluidine 121-72-2 C9H13N 135.209 —— 1-azido-3-methylbenzene 4113-72-8 C7H7N3 133.153 4-碘-3-甲基苯胺 4-iodo-3-methylaniline 4949-69-3 C7H8IN 233.052 3,3'-二甲基联苯胺 3,3'-ditolylamine 626-13-1 C14H15N 197.28 3-甲基二苯胺 3-Methyldiphenylamine 1205-64-7 C13H13N 183.253 2-碘-5-甲基苯胺 2-iodo-5-methylaniline 13194-69-9 C7H8IN 233.052 2-氟-5-甲基苯胺 3-amino-4-fluorotoluene 452-84-6 C7H8FN 125.146 2-甲基-4-氨基苯酚 4-amino-2-methylphenol 2835-96-3 C7H9NO 123.155 4-氯-3-甲基苯胺 3-methyl-4-chloroaniline 7149-75-9 C7H8ClN 141.6 2-溴-5-甲基苯胺 2-bromo-5-methylaniline 53078-85-6 C7H8BrN 186.051 4-溴-3-甲基苯胺 amino-5-bromo-2-toluene 6933-10-4 C7H8BrN 186.051 —— 1-phenyl-2-(m-tolyl)hydrazine 621-25-0 C13H14N2 198.268 —— 3,3'-dimethylhydrazobenzene 621-26-1 C14H16N2 212.294 —— trans-3,3'-dimethylazobenzene 51437-67-3 C14H14N2 210.279 3,3'-偶氮甲苯 3,3'-dimethylazobenzene 588-04-5 C14H14N2 210.279 —— phenyl-m-tolyl-diazene 17478-66-9 C13H12N2 196.252 —— N-(2-aminoethyl)-3-methylaniline 14088-79-0 C9H14N2 150.224 2-乙基-5-甲基苯胺 2-ethyl-5-methylaniline 4748-81-6 C9H13N 135.209 —— 4-ethyl-3-methylaniline 69450-94-8 C9H13N 135.209 N-异丙基间甲苯胺 N-isopropyl-3-methylaniline 10219-26-8 C10H15N 149.236 丙基-间甲苯胺 N-propyl-3-methylaniline 142031-46-7 C10H15N 149.236 —— N-<3-Methyl-phenyl>-β-mercapto-aethylamin 91267-25-3 C9H13NS 167.275 —— 3-methyl-N-(p-tolyl)aniline 62121-57-7 C14H15N 197.28 —— N-allyl-3-methylaniline 15258-45-4 C10H13N 147.22 —— 1-(3-tolyl)guanidine 45954-03-8 C8H11N3 149.195 4-氨基-2-甲基苯甲醛 2-methyl-4-aminobenzaldehyde 61594-81-8 C8H9NO 135.166 N-羟乙基-间甲基苯胺 1-(2-hydroxyethyl)amino-3-methylbenzene 102-41-0 C9H13NO 151.208 —— 5-methyl-2-vinylaniline 143879-61-2 C9H11N 133.193 —— N-(Methoxymethyl)-m-anisidine 88919-93-1 C9H13NO 151.208 —— bis(3-methylphenylamino)methane 115162-95-3 C15H18N2 226.321 4-氨基-2-甲基苄腈 4-amino-2-methylbenzonitrile 72115-06-1 C8H8N2 132.165 2-碘-3-甲基苯胺 2-iodo-3-methylaniline 89938-16-9 C7H8IN 233.052 —— N1-(m-tolyl)benzene-1,4-diamine 42771-90-4 C13H14N2 198.268 2-氨基-4-甲基-N-甲基氨基苯 2-amino-4-methyl-N-methylaminobenzene 39513-19-4 C8H12N2 136.197 —— 4-nitroso-3-methylaniline 91599-63-2 C7H8N2O 136.153 2-氨基-4-甲基苯甲腈 5-methyl-2-cyanoaniline 26830-96-6 C8H8N2 132.165 - 1
- 2
- 3
- 4
- 5
- 6
反应信息
-
作为反应物:描述:3-甲基苯胺 在 盐酸 、 copper(I) sulfate 、 arsenic(III) trioxide 、 sodium carbonate 、 sodium nitrite 作用下, 生成 3-甲基苯基砷酸参考文献:名称:Yambushev, F. D.; Kovyrzina, V>; P., Journal of general chemistry of the USSR, 1981, p. 1919 - 1926摘要:DOI:
-
作为产物:参考文献:名称:使用Pd / C进行直接液相苯酚-苯胺胺化†摘要:在这里,我们报告了苯酚与氨的直接直接胺化反应在液相中生成的苯胺。为此,Pd / C被证明是最合适的催化剂。反应参数的优化导致伯胺的选择性生产和可持续生产,收率高达95%。DOI:10.1039/c8cy00193f
-
作为试剂:描述:参考文献:名称:Heiduschka, Journal fur praktische Chemie (Leipzig 1954), 1910, vol. <2>81, p. 321摘要:DOI:
文献信息
-
Synthesis and Biological Evaluation of Novel L-Homoserine Lactone Analogs as Quorum Sensing Inhibitors of <i>Pseudomonas aeruginosa</i>作者:Haoyue Liu、Qianhong Gong、Chunying Luo、Yongxi Liang、Xiaoyan Kong、Chunli Wu、Pengxia Feng、Qing Wang、Hui Zhang、M.A. WirekoDOI:10.1248/cpb.c19-00359日期:2019.10.1series of novel L-homoserine lactone analogs and evaluated their in vitro quorum sensing (QS) inhibitory activity against two biomonitor strains, Chromobacterium violaceum CV026 and Pseudomonas aeruginosa PAO1. Studies of the structure-activity relationships of the set of L-homoserine lactone analogs indicated that phenylurea-containing N-dithiocarbamated homoserine lactones are more potent than (Z)-
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
Highly Regio- and Enantioselective Alkoxycarbonylative Amination of Terminal Allenes Catalyzed by a Spiroketal-Based Diphosphine/Pd(II) Complex作者:Jiawang Liu、Zhaobin Han、Xiaoming Wang、Zheng Wang、Kuiling DingDOI:10.1021/jacs.5b07764日期:2015.12.16An enantioselective alkoxycarbonylation-amination cascade process of terminal allenes with CO, methanol, and arylamines has been developed. It proceeds under mild conditions (room temperature, ambient pressure CO) via oxidative Pd(II) catalysis using an aromatic spiroketal-based diphosphine (SKP) as a chiral ligand and a Cu(II) salt as an oxidant and affords a wide range of α-methylene-β-arylamino
-
Dihydroquinazolines enhance 20S proteasome activity and induce degradation of α-synuclein, an intrinsically disordered protein associated with neurodegeneration作者:Taylor J. Fiolek、Christina L. Magyar、Tyler J. Wall、Steven B. Davies、Molly V. Campbell、Christopher J. Savich、Jetze J. Tepe、R. Adam MoseyDOI:10.1016/j.bmcl.2021.127821日期:2021.3traditional small molecule drug design and are often referred to as “undruggable”. The 20S proteasome is the main protease that targets IDPs for degradation and therefore small molecule 20S proteasome enhancement presents a novel therapeutic strategy by which these undruggable IDPs could be targeted. The concept of 20S activation is still relatively new, with few potent activators having been identified许多内在无序蛋白质 (IDP) 的聚集体或寡聚形式,包括 α-突触核蛋白,是帕金森病和阿尔茨海默病等神经退行性疾病的标志,也是其发病机制的关键因素。由于其无序的性质,因此缺乏明确的药物结合口袋,IDPs 是传统小分子药物设计的困难目标,通常被称为“不可药物”。20S 蛋白酶体是靶向 IDP 进行降解的主要蛋白酶,因此小分子 20S 蛋白酶体增强提供了一种新的治疗策略,通过该策略可以靶向这些不可成药的 IDP。20S 激活的概念仍然相对较新,迄今为止已确定的有效激活剂很少。在此处,我们合成并评估了一个二氢喹唑啉类似物库,并发现了几种有前景的新型 20S 蛋白酶体激活剂。对热门歌曲的进一步测试表明,它们可以增强 20S 介导的 α-突触核蛋白降解,这是与帕金森病相关的 IDP。
-
Novel compounds and compositions as protease inhibitors申请人:——公开号:US20020052378A1公开(公告)日:2002-05-02The present invention relates to novel cysteine protease inhibitors of Formula I: 1 the pharmaceutically acceptable salts and N-oxide derivatives thereof, their use as therapeutic agents and methods of making them.
表征谱图
-
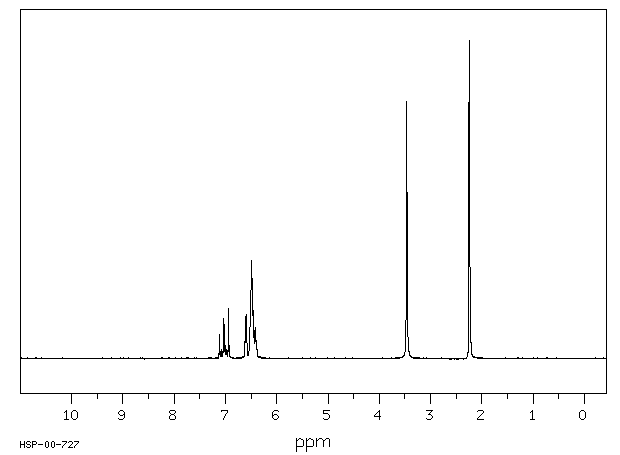
氢谱1HNMR
-
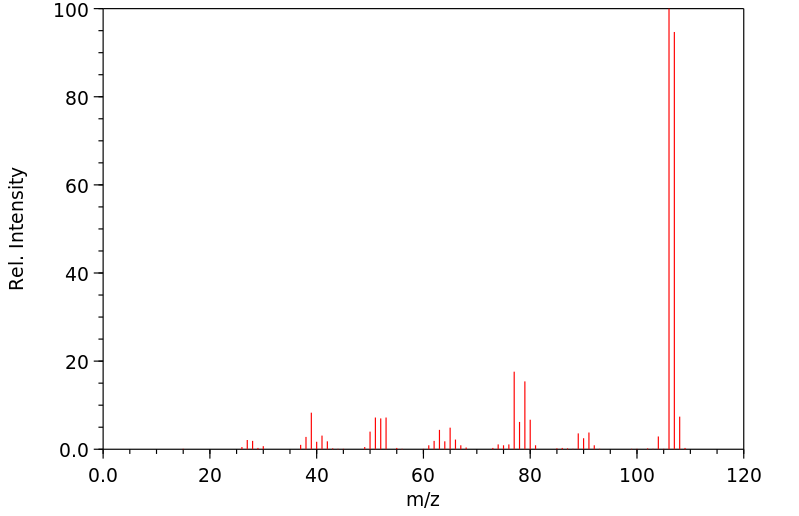
质谱MS
-
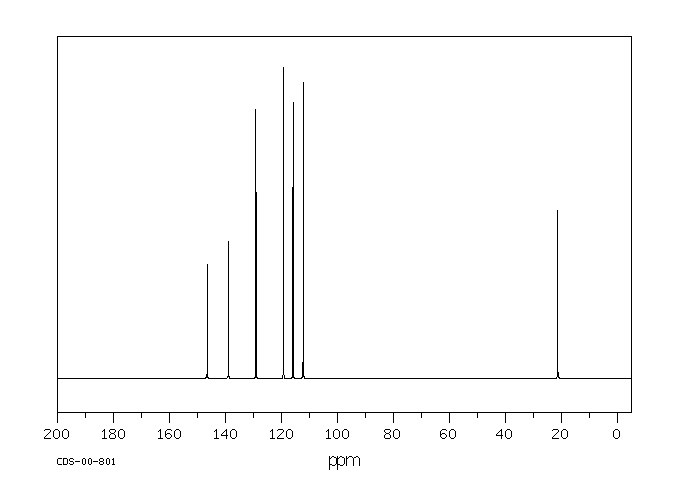
碳谱13CNMR
-
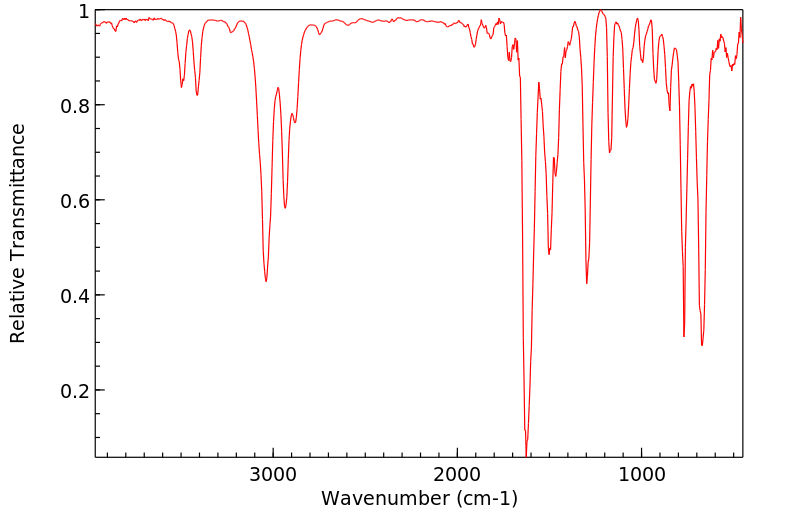
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息